Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice
- PMID: 10878107
- PMCID: PMC2269993
- DOI: 10.1111/j.1469-7793.2000.00143.x
Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice
Abstract
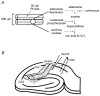
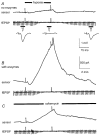
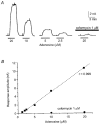
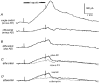
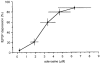
We have used an enzyme-based, twin-barrelled sensor to measure adenosine release during hypoxia in the CA1 region of rat hippocampal slices in conjunction with simultaneous extracellular field recordings of excitatory synaptic transmission. When loaded with a combination of adenosine deaminase, nucleoside phosphorylase and xanthine oxidase, the sensor responded linearly to exogenous adenosine over the concentration range 10 nM to 20 microM. Without enzymes, the sensor when placed on the surface of hippocampal slices recorded a very small net signal during hypoxia of 40 +/- 43 pA (mean +/- s.e.m.; n = 7). Only when one barrel was loaded with the complete sequence of enzymes and the other with the last two in the cascade did the sensor record a large net difference signal during hypoxia (1226 +/- 423 pA; n = 7). This signal increased progressively during the hypoxic episode, scaled with the hypoxic depression of the simultaneously recorded field excitatory postsynaptic potential and was greatly reduced (67 +/- 6.5 %; n = 9) by coformycin (0.5-2 microM), a selective inhibitor of adenosine deaminase, the first enzyme in the enzymic cascade within the sensor. For 5 min hypoxic episodes, the sensor recorded a peak concentration of adenosine of 5.6 +/- 1.2 microM (n = 16) with an IC(50) for the depression of transmission of approximately 3 microM. In slices pre-incubated for 3-6 h in nominally Ca(2+)-free artificial cerebrospinal fluid, 5 min of hypoxia resulted in an approximately 9-fold greater release of adenosine (48.9 +/- 17.7 microM; n = 6). High extracellular Ca(2+) (4 mM) both reduced the adenosine signal recorded by the sensor during hypoxia (3.5 +/- 0.6 microM; n = 4) and delayed the hypoxic depression of excitatory synaptic transmission.
Figures



References
-
- Anderson WW, Collingridge GL. A data acquisition program for on-line analysis of long-term potentiation and long-term depression. Society for Neuroscience Abstracts. 1997;23:665. - PubMed
-
- Arlinghaus L, Lee KS. Endogenous adenosine mediates the sustained inhibition of excitatory synaptic transmission during moderate hypoxia. Brain Research. 1996;724:265–268. - PubMed
-
- Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Advances in Pharmacology. 1997;39:353–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

