Effect of pulmonary C-fibre afferent stimulation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats
- PMID: 10878108
- PMCID: PMC2269989
- DOI: 10.1111/j.1469-7793.2000.t01-1-00157.x
Effect of pulmonary C-fibre afferent stimulation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats
Abstract
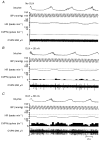
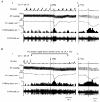
It has been demonstrated previously that the vagal bradycardia evoked by activation of pulmonary C-fibres is not respiratory modulated. Experiments were carried out in alpha-chloralose anaesthetized cats to determine if these cardiac vagal preganglionic neurones (CVPNs) in the nucleus ambiguus (NA), which have respiratory modulated activity, can be activated when pulmonary C-fibre afferents are stimulated by right atrial injections of phenylbiguanide (PBG). Eleven CVPNs with B-fibre axons in the right cardiac vagal branches were identified and found to be localized within or ventrolateral to the nucleus ambiguus. Ionophoretic application of a high current of dl-homocysteic acid (DLH) induced a vagally mediated bradycardia and hypotension in six of eight sites from which CVPNs were recorded. The activity of B-fibre CVPNs, whether spontaneous (n = 4) or induced by ionophoresis of DLH (n = 7) was respiratory modulated, firing perferentially during post-inspiration and stage 2 expiration. This activity also correlated with the rising phase of the arterial blood pressure wave consistent with these CVPNs receiving an arterial baroreceptor input. Right atrial injections of PBG excited nine of eleven CVPNs tested. In eight of these activated neurones the onset latency of the excitation was within the pulmonary circulation time, consistent with being activated only by pulmonary C-fibre afferents. In two neurones the PBG-evoked excitation still occurred when central inspiratory drive was inhibited, as indicated by the disappearance of phrenic nerve activity. In conclusion, B-fibre respiratory modulated CVPNs can be activated following stimulation of pulmonary C-fibre afferents.
Figures



References
-
- Berman AL. The Brainstem of the Cat. Madison, WI, USA: University of Wisconsin; 1968.
-
- Coleridge JCG, Coleridge HM. Handbook of Physiology, section 2, The Cardiovascular System, The Heart. Vol. 1. Bethesda, MD, USA: American Physiological Society; 1979. Chemoreflex regulation of the heart; pp. 653–676.
-
- Coleridge JCG, Coleridge HM. Afferent vagal C-fibre innervation of the lungs and airways and its function significance. Reviews of Physiology, Biochemistry and Pharmacology. 1984;99:1–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

