Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys
- PMID: 10880046
- PMCID: PMC314358
- DOI: 10.1172/JCI9102
Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys
Abstract
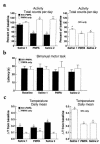
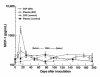
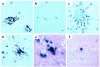
Simian immunodeficiency virus (SIV) infection of rhesus monkeys provides an excellent model of the central nervous system (CNS) consequences of HIV infection. To discern the relationship between viral load and abnormalities induced in the CNS by the virus, we infected animals with SIV and later instituted antiviral treatment to lower peripheral viral load. Measurement of sensory-evoked potentials, assessing CNS neuronal circuitry, revealed delayed latencies after infection that could be reversed by lowering viral load. Cessation of treatment led to the reappearance of these abnormalities. In contrast, the decline in general motor activity induced by SIV infection was unaffected by antiviral treatment. An acute increase in the level of the chemokine monocyte chemoattractant protein-1 (MCP-1) was found in the cerebrospinal fluid (CSF) relative to plasma in the infected animals at the peak of acute viremia, likely contributing to an early influx of immune cells into the CNS. Examination of the brains of the infected animals after return of the electrophysiological abnormalities revealed diverse viral and inflammatory findings. Although some of the physiological abnormalities resulting from SIV infection can be at least temporarily reversed by lowering viral load, the viral-host interactions initiated by infection may result in long-lasting changes in CNS-mediated functions.
Figures

Comment in
-
HIV and HIV dementia.J Clin Invest. 2000 Jul;106(1):11-3. doi: 10.1172/JCI10553. J Clin Invest. 2000. PMID: 10880043 Free PMC article. No abstract available.
References
-
- Price RW, et al. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. - PubMed
-
- Heaton RK, et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1994;56:8–17. - PubMed
-
- Michaels SH, Clark R, Kissinger P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;339:405–406. - PubMed
-
- Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;14:1933–1942. - PubMed
-
- Dore GJ, et al. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

