Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation
- PMID: 10880051
- PMCID: PMC314357
- DOI: 10.1172/JCI8706
Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation
Abstract
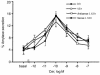
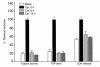
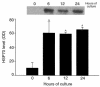
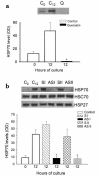
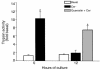
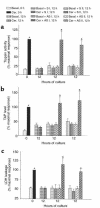
Rodents given a supramaximally stimulating dose of cholecystokinin or its analogue cerulein develop acute pancreatitis with acinar cell injury, pancreatic inflammation, and intrapancreatic digestive enzyme (i.e., trypsinogen) activation. Prior thermal stress is associated with heat shock protein 70 (HSP70) expression and protection against cerulein-induced pancreatitis. However, thermal stress can also induce expression of other HSPs. The current studies were performed using an in vitro system to determine whether HSP70 can actually mediate protection against pancreatitis and, if so, to define the mechanism underlying that protection. We show that in vitro exposure of freshly prepared rat pancreas fragments to a supramaximally stimulating dose of cerulein results in changes similar to those noted in cerulein-induced pancreatitis, i.e., intra-acinar cell trypsinogen activation and acinar cell injury. Short-term culture of the fragments results in HSP70 expression and loss of the pancreatitis-like changes noted after addition of cerulein. The culture-induced enhanced HSP70 expression can be prevented by addition of either the flavonoid antioxidant quercetin or an antisense oligonucleotide to HSP70. Under these latter conditions, addition of a supramaximally stimulating concentration of cerulein results in trypsinogen activation and acinar cell injury. These findings indicate that the protection against cerulein-induced pancreatitis that follows culture-induced (and possibly thermal) stress is mediated by HSP70. They suggest that the HSP acts by preventing trypsinogen activation within acinar cells.
Figures


References
-
- Lampel M, Kern HF. Acute interstitial pancreatitis in rats induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–113. - PubMed
-
- Grady T, Saluja A, Kaiser A, Steer M. Edema and intrapancreatic trypsinogen activation precede glutathione depletion during caerulein pancreatitis. Am J Physiol. 1996;271:G20–G26. - PubMed
-
- Saluja AK, et al. Caerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–310. - PubMed
-
- Hofbauer B, et al. Intra-acinar activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352–G362. - PubMed
-
- Saluja AK, et al. Secretagogue-induced digestive enzyme activation and cell injury in pancreatic acini. Am J Physiol. 1999;276:G835–G842. - PubMed

