Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy
- PMID: 10880055
- PMCID: PMC314354
- DOI: 10.1172/JCI6218
Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy
Abstract
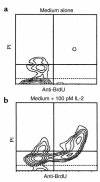
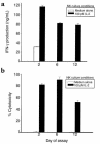
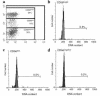
The continuous, in vivo infusion of low-dose IL-2 selectively expands the absolute number of human natural killer (NK) cells after 4-6 weeks of therapy. The mechanism responsible for this expansion is unknown and was examined in this study. NK cells cultured at low concentrations of IL-2, comparable to those found during in vivo therapy, proliferate for 6 days and then exit the cell cycle. However, NK cells in vivo did not traverse the S/G(2)/M phase of the cell cycle during low-dose IL-2 therapy. Low concentrations of IL-2 delay programmed cell death of NK cells but have the same effect on resting T cells that do not expand in vivo. When CD34(+) bone marrow hematopoietic progenitor cells are cultured for 21 days with low concentrations of IL-2, they differentiate into CD56(+)CD3(-) NK cells, not T cells. Thus, the selective expansion of human NK cells during continuous in vivo infusion of low-dose IL-2 likely results from enhanced NK-cell differentiation from bone marrow progenitors, combined with an IL-2-dependent delay in NK-cell death, rather than proliferation of mature NK cells in the periphery.
Figures


References
-
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

