The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis
- PMID: 10880374
- PMCID: PMC1850214
- DOI: 10.1016/S0002-9440(10)64515-4
The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis
Abstract
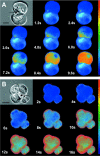
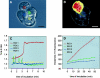
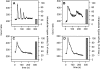
The exocrine pancreas synthesizes and secretes large amounts of digestive proteases as inactive precursor zymogens. Under physiological conditions a variety of cellular defense mechanisms protect the pancreatic acinar cell against a premature and intracellular activation of these zymogens. When these defenses fail, pancreatic autodigestion is initiated and acute pancreatitis can develop. A number of experimental observations suggest that extra- as well as intracellular calcium concentrations play an important part in the initiation of pancreatic protease activation, but the intracellular signaling events that regulate this process are unknown. Using a model system in which we used pancreatic acini (freshly prepared functional units of living acinar cells), we were able to simulate the conditions found during experimental pancreatitis in rodents. By means of a cell permeant fluorescent trypsin substrate we could demonstrate in these acini that premature protease activation is initiated at the apical acinar cell pole and occurs only in the presence of secretagogue concentrations that exceed those required for a maximum secretory response. By combining this technique with fluorescence ratio imaging for the Ca(2+)-sensitive dye fura-2, we could further show that this protease activation is highly dependent on the spatial as well as the temporal distribution of the corresponding Ca(2+) release from stores within the same subcellular compartment and that it is not propagated to neighboring acinar cells.
Figures


References
-
- Chiari H: Über die Selbstverdauung des menschlichen Pankreas. Z Heilk 1896, 17:69-96
-
- Whitcomb DC: Early trypsinogen activation in acute pancreatitis. Gastroenterology 1999, 116:770-772 - PubMed
-
- Mithofer K, Fernandez-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL: Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology 1995, 109:239–246 - PubMed
-
- Ward JB, Peterson OH, Jenkins SA, Sutton R: Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet 1995, 346:1016-1019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

