Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy
- PMID: 10880382
- PMCID: PMC1850216
- DOI: 10.1016/S0002-9440(10)64523-3
Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy
Abstract
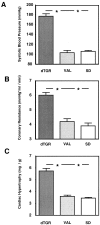
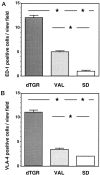
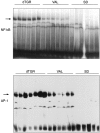
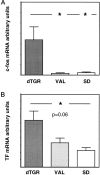
Tissue factor (TF), a main initiator of clotting, is up-regulated in vasculopathy. We tested the hypothesis that chronic in vivo angiotensin (ANG) II receptor AT(1) receptor blockade inhibits TF expression in a model of ANG II-induced cardiac vasculopathy. Furthermore, we explored the mechanisms by examining transcription factor activation and analyzing the TF promoter. Untreated transgenic rats overexpressing the human renin and angiotensinogen genes (dTGR) feature hypertension and severe left ventricular hypertrophy with focal areas of necrosis, and die at age 7 weeks. Plasma and cardiac ANG II was three- to fivefold increased compared to Sprague-Dawley rats. Chronic treatment with valsartan normalized blood pressure and coronary resistance completely, and ameliorated cardiac hypertrophy (P < 0.001). Valsartan prevented monocyte/macrophage infiltration, nuclear factor-kappaB (NF-kappaB) and activator protein-1 (AP-1) activation, and c-fos expression in dTGR hearts. NF-kappaB subunit p65 and TF expression was increased in the endothelium and media of cardiac vessels and markedly reduced by valsartan treatment. To analyze the mechanism of TF transcription, we then transfected human coronary artery smooth muscle cells and Chinese hamster ovary cells overexpressing the AT(1) receptor with plasmids containing the human TF promoter and the luciferase reporter gene. ANG II induced the full-length TF promoter in both transfected cell lines. TF transcription was abolished by AT(1) receptor blockade. Deletion of both AP-1 and NF-kappaB sites reduced ANG II-induced TF gene transcription completely, whereas the deletion of AP-1 sites reduced transcription. Thus, the present study clearly shows an aberrant TF expression in the endothelium and media in rats with ANG II-induced vasculopathy. The beneficial effects of AT(1) receptor blockade in this model are mediated via the inhibition of NF-kappaB and AP-1 activation, thereby preventing TF expression, cardiac vasculopathy, and microinfarctions.
Figures




Comment in
-
Association between the molecular pathobiology of essential hypertension and thrombotic diseases.Am J Pathol. 2000 Jul;157(1):5-6. doi: 10.1016/S0002-9440(10)64509-9. Am J Pathol. 2000. PMID: 10880368 Free PMC article. No abstract available.
Similar articles
-
Effect of bosentan on NF-kappaB, inflammation, and tissue factor in angiotensin II-induced end-organ damage.Hypertension. 2000 Aug;36(2):282-90. doi: 10.1161/01.hyp.36.2.282. Hypertension. 2000. PMID: 10948091
-
Angiotensin II induces monocyte chemoattractant protein-1 expression via a nuclear factor-kappaB-dependent pathway in rat preadipocytes.Am J Physiol Endocrinol Metab. 2006 Oct;291(4):E771-8. doi: 10.1152/ajpendo.00560.2005. Epub 2006 May 16. Am J Physiol Endocrinol Metab. 2006. PMID: 16705055
-
Cerivastatin prevents angiotensin II-induced renal injury independent of blood pressure- and cholesterol-lowering effects.Kidney Int. 2000 Oct;58(4):1420-30. doi: 10.1046/j.1523-1755.2000.00304.x. Kidney Int. 2000. PMID: 11012877
-
[Pathophysiological and clinical implications of AT(1) and AT(2) angiotensin II receptors in metabolic disorders: hypercholesterolaemia and diabetes].Drugs. 2002;62 Spec No 1:31-41. Drugs. 2002. PMID: 12036387 Review. French.
-
Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor.Mol Cell Biochem. 2000 Sep;212(1-2):155-69. Mol Cell Biochem. 2000. PMID: 11108147 Review.
Cited by
-
Differential induction of cellular proliferation, hypertrophy and apoptosis in H9c2 cardiomyocytes by exogenous tissue factor.Mol Cell Biochem. 2010 Dec;345(1-2):119-30. doi: 10.1007/s11010-010-0565-8. Epub 2010 Aug 22. Mol Cell Biochem. 2010. PMID: 20730477
-
Autoantibodies Targeting AT1- and ETA-Receptors Link Endothelial Proliferation and Coagulation via Ets-1 Transcription Factor.Int J Mol Sci. 2021 Dec 27;23(1):244. doi: 10.3390/ijms23010244. Int J Mol Sci. 2021. PMID: 35008670 Free PMC article.
-
The Role of Traditional Chinese Medicine in the Regulation of Oxidative Stress in Treating Coronary Heart Disease.Oxid Med Cell Longev. 2019 Feb 24;2019:3231424. doi: 10.1155/2019/3231424. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30918578 Free PMC article. Review.
-
Mechanisms in hypertension and target organ damage: Is the role of the thymus key? (Review).Int J Mol Med. 2018 Jul;42(1):3-12. doi: 10.3892/ijmm.2018.3605. Epub 2018 Mar 30. Int J Mol Med. 2018. PMID: 29620247 Free PMC article. Review.
-
Tissue factor, protease activated receptors and pathologic heart remodelling.Thromb Haemost. 2014 Nov;112(5):893-900. doi: 10.1160/TH14-03-0243. Epub 2014 Aug 7. Thromb Haemost. 2014. PMID: 25104210 Free PMC article. Review.
References
-
- Edgington TS, Mackman N, Brand K, Ruf W: The structural biology of expression and function of tissue factor. Thromb Haemost 1991, 66:67-79 - PubMed
-
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D: Role of tissue factor in embryonic blood vessel development. Nature 1996, 383:73-75 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

