The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system
- PMID: 10880388
- PMCID: PMC1850221
- DOI: 10.1016/S0002-9440(10)64529-4
The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system
Abstract
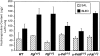
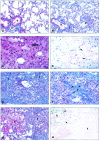
Acute and chronic pulmonary diseases are characterized by impaired fibrinolytic activity within the lung. To determine the role of the fibrinolytic system in regulating the pathologies associated with lung injury, we examined the effect of bleomycin, an agent that induces the development of pulmonary fibrosis, in mice deficient for plasminogen (Pg(-)(/-)), urokinase (u-PA(-)(/-)), urokinase receptor (u-PAR(-)(/-)), or tissue plasminogen activator (t-PA(-)(/-)), and in control wild-type (WT) mice. Pg(-)(/-) and t-PA(-)(/-) mice demonstrated an enhanced increase in lung collagen content relative to that observed in WT mice. Levels in u-PA(-)(/-) and u-PAR(-)(/-) mice were similar to those in WT mice. Histological analysis 14 days after lung injury confirmed enhanced interstitial fibrosis in Pg(-)(/-), u-PA(-)(/-), and t-PA(-)(/-) mice relative to WT and u-PAR(-)(/-) mice. Areas of pulmonary hemorrhage were observed in bleomycin-treated WT mice and not in Pg(-)(/-), u-PA(-)(/-), and u-PAR(-)(/-) mice or saline controls. Instead, extensive areas of fibrosis were present throughout the lungs of bleomycin-treated Pg(-)(/-) and u-PA(-)(/-) mice. A mixed phenotype (hemorrhage and fibrosis) was observed in t-PA(-)(/-) and Pg(+/-) mice. Hemosiderin-laden macrophages were abundant in the lungs of mice exhibiting hemorrhage and these mice were prone to an early death. Enhanced macrophage levels in the lungs and activation of matrix metalloelastase (MMP-12) were found in mice with a hemorrhage phenotype. The results of these studies indicate a role for the fibrinolytic system in acute lung injury and suggests that intra-alveolar hemorrhage is the result of basement membrane degradation through cell-mediated u-PA activation of Pg with possible involvement of matrix metalloproteinases. Absence of these two components of the fibrinolytic system, either urokinase or plasminogen, results in accelerated fibrosis.
Figures





Similar articles
-
Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice.J Clin Invest. 2000 Dec;106(11):1341-50. doi: 10.1172/JCI10531. J Clin Invest. 2000. PMID: 11104787 Free PMC article.
-
Deficiency of urokinase-type plasminogen activator-mediated plasmin generation impairs vascular remodeling during hypoxia-induced pulmonary hypertension in mice.Circulation. 2001 Apr 17;103(15):2014-20. doi: 10.1161/01.cir.103.15.2014. Circulation. 2001. PMID: 11306532
-
Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse.J Clin Invest. 1995 Sep;96(3):1621-30. doi: 10.1172/JCI118201. J Clin Invest. 1995. PMID: 7544811 Free PMC article.
-
The fibrinolytic system in neoplasia.Semin Thromb Hemost. 1996;22(6):459-78. doi: 10.1055/s-2007-999047. Semin Thromb Hemost. 1996. PMID: 9122711 Review.
-
Elements of the fibrinolytic system.Ann N Y Acad Sci. 2001;936:226-36. doi: 10.1111/j.1749-6632.2001.tb03511.x. Ann N Y Acad Sci. 2001. PMID: 11460480 Review.
Cited by
-
miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1.PLoS Genet. 2013;9(2):e1003291. doi: 10.1371/journal.pgen.1003291. Epub 2013 Feb 14. PLoS Genet. 2013. PMID: 23459460 Free PMC article.
-
Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice.Am J Respir Cell Mol Biol. 2006 Sep;35(3):289-97. doi: 10.1165/rcmb.2005-0471OC. Epub 2006 Mar 30. Am J Respir Cell Mol Biol. 2006. PMID: 16574944 Free PMC article.
-
The effects of intrinsic pathway protease deficiencies on plasminogen-deficient mice.Blood. 2005 Nov 1;106(9):3055-7. doi: 10.1182/blood-2005-02-0577. Epub 2005 Jun 28. Blood. 2005. PMID: 15985536 Free PMC article.
-
Genetically manipulated mouse models of lung disease: potential and pitfalls.Am J Physiol Lung Cell Mol Physiol. 2012 Mar 15;302(6):L485-97. doi: 10.1152/ajplung.00085.2011. Epub 2011 Dec 23. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22198907 Free PMC article. Review.
-
Genes involved in innate immunity associated with asbestos-related fibrotic changes.Occup Environ Med. 2014 Jan;71(1):48-54. doi: 10.1136/oemed-2013-101555. Epub 2013 Oct 4. Occup Environ Med. 2014. PMID: 24142982 Free PMC article.
References
-
- National Center for Health Statistics: Births and deaths: United States. Monthly Vital Statistics Report. Atlanta, Centers for Disease Control, 1997, vol. 46, no. 1, suppl. 2 - PubMed
-
- Katzenstein A-L, Askin FB: Idiopathic interstitial pneumonia/idiopathic pulmonary fibrosis. Surgical Pathology of Non-Neoplastic Lung Disease. 1990, :pp 58-96 W.B. Saunders/Harcourt Brace Jovanovich, Inc., Philadelphia
-
- Katzenstein A-L, Askin FB: Acute lung injury patterns: diffuse alveolar damage, acute interstial pneumonia, bronchiolitis obliterans-organizing pneumonia. Surgical Pathology of Non-Neoplastic Lung Disease. 1990, :pp 9-57 W. B. Saunders/Harcourt Brace Jovanovich, Inc., Philadelphia
-
- Crouch E: Pathology of pulmonary fibrosis. Am J Physiol 1990, 259:L159-L184 - PubMed
-
- Tomashefski JF, Jr: Pathology of Pulmonary Disease. 1994:pp 125-138 J. B. Lippincott Company, Edited by MJ Saldana. Philadelphia
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

