Disruption of redox homeostasis in tumor necrosis factor-induced apoptosis in a murine hepatocyte cell line
- PMID: 10880392
- PMCID: PMC1850198
- DOI: 10.1016/S0002-9440(10)64533-6
Disruption of redox homeostasis in tumor necrosis factor-induced apoptosis in a murine hepatocyte cell line
Abstract
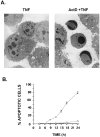
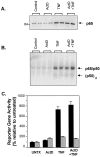
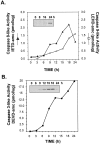
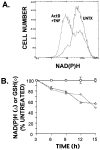
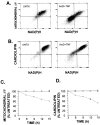
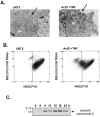
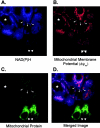
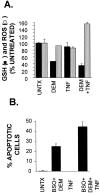
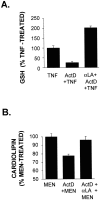
Tumor necrosis factor (TNF) is a mediator of the acute phase response in the liver and can initiate proliferation and cause cell death in hepatocytes. We investigated the mechanisms by which TNF causes apoptosis in hepatocytes focusing on the role of oxidative stress, antioxidant defenses, and mitochondrial damage. The studies were conducted in cultured AML12 cells, a line of differentiated murine hepatocytes. As is the case for hepatocytes in vivo, AML12 cells were not sensitive to cell death by TNF alone, but died by apoptosis when exposed to TNF and a small dose of actinomycin D (Act D). Morphological signs of apoptosis were not detected until 6 hours after the treatment and by 18 hours approximately 50% of the cells had died. Exposure of the cells to TNF+Act D did not block NFkappaB nuclear translocation, DNA binding, or its overall transactivation capacity. Induction of apoptosis was characterized by oxidative stress indicated by the loss of NAD(P)H and glutathione followed by mitochondrial damage that included loss of mitochondrial membrane potential, inner membrane structural damage, and mitochondrial condensation. These changes coincided with cytochrome C release and the activation of caspases-8, -9, and -3. TNF-induced apoptosis was dependent on glutathione levels. In cells with decreased levels of glutathione, TNF by itself in the absence of transcriptional blocking acted as an apoptotic agent. Conversely, the antioxidant alpha-lipoic acid, that protected against the loss of glutathione in cells exposed to TNF+Act D completely prevented mitochondrial damage, caspase activation, cytochrome C release, and apoptosis. The results demonstrate that apoptosis induced by TNF+Act D in AML12 cells involves oxidative injury and mitochondrial damage. As injury was regulated to a larger extent by the glutathione content of the cells, we suggest that the combination of TNF+Act D causes apoptosis because Act D blocks the transcription of genes required for antioxidant defenses.
Figures
References
-
- Ashkenazi A, Dixit VM: Death receptors: signaling and modulation. Science 1998, 281:1305-1308 - PubMed
-
- Wallach D, Boldin M, Varfolomeev E, Beyaert R, Vandenabeele P, Fiers W: Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett 1997, 410:96-106 - PubMed
-
- Arch RH, Gedrich RW, Thompson CB: Tumor necrosis factor receptor-associated factors (TRAFs): a family of adapter proteins that regulates life and death. Genes Dev 1998, 12:2821-2830 - PubMed
-
- Ghosh S, May MJ, Kopp EB. NFκB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998, 16:225–260 - PubMed
-
- Hsu H, Xiong J, Goeddel D: The TNF receptor 1-associated protein TRADD signals cell death and NFκB activation. Cell 1995, 81:495-504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

