Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations
- PMID: 10880397
- PMCID: PMC1850219
- DOI: 10.1016/s0002-9440(10)64538-5
Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations
Abstract
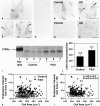
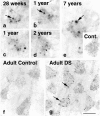
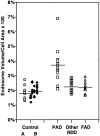
Endocytosis is critical to the function and fate of molecules important to Alzheimer's disease (AD) etiology, including the beta protein precursor (betaPP), amyloid beta (Abeta) peptide, and apolipoprotein E (ApoE). Early endosomes, a major site of Abeta peptide generation, are markedly enlarged within neurons in the Alzheimer brain, suggesting altered endocytic pathway (EP) activity. Here, we show that neuronal EP activation is a specific and very early response in AD. To evaluate endocytic activation, we used markers of internalization (rab5, rabaptin 5) and recycling (rab4), and found that enlargement of rab5-positive early endosomes in the AD brain was associated with elevated levels of rab4 immunoreactive protein and translocation of rabaptin 5 to endosomes, implying that both endocytic uptake and recycling are activated. These abnormalities were evident in pyramidal neurons of the neocortex at preclinical stages of disease when Alzheimer-like neuropathology, such as Abeta deposition, was restricted to the entorhinal region. In Down syndrome, early endosomes were significantly enlarged in some pyramidal neurons as early as 28 weeks of gestation, decades before classical AD neuropathology develops. Markers of EP activity were only minimally influenced by normal aging and other neurodegenerative diseases studied. Inheritance of the epsilon4 allele of APOE, however, accentuated early endosome enlargement at preclinical stages of AD. By contrast, endosomes were normal in size at advanced stages of familial AD caused by mutations of presenilin 1 or 2, indicating that altered endocytosis is not a consequence of Abeta deposition. These results identify EP activation as the earliest known intraneuronal change to occur in sporadic AD, the most common form of AD. Given the important role of the EP in Abeta peptide generation and ApoE function, early endosomal abnormalities provide a mechanistic link between EP alterations, genetic susceptibility factors, and Abeta generation and suggest differences that may be involved in Abeta generation and beta amyloidogenesis in subtypes of AD.
Figures

References
-
- Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM, Doms RW: Alzheimer’s A beta(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med 1997, 3:1021-1023 - PubMed
-
- Hartmann H, Busciglio J, Baumann KH, Staufenbiel M, Yankner BA: Developmental regulation of presenilin-1 processing in the brain suggests a role in neuronal differentiation. J Biol Chem 1997, 272:14505-14508 - PubMed
-
- Xia W, Zhang J, Ostaszewski BL, Kimberly WT, Seubert P, Koo EH, Shen J, Selkoe DJ: Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi. Biochemistry 1998, 37:16465-16471 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

