Intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and regulated on activation normal T cell expressed and secreted are expressed by human breast carcinoma cells and support eosinophil adhesion and activation
- PMID: 10880401
- PMCID: PMC1850201
- DOI: 10.1016/S0002-9440(10)64542-7
Intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and regulated on activation normal T cell expressed and secreted are expressed by human breast carcinoma cells and support eosinophil adhesion and activation
Abstract
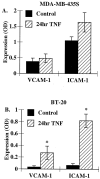
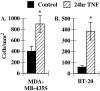
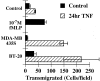
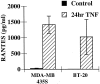
Eosinophils are usually associated with parasitic and allergic diseases; however, eosinophilia is also observed in several types of human tumors, including breast carcinomas. In this study we examined several human breast carcinoma cell lines for adhesion molecule expression and the ability to bind and activate eosinophils. MDA-MB-435S and MDA-MB-468 cells constitutively expressed both intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and this expression was enhanced by treatment with tumor necrosis factor-alpha (TNF-alpha). BT-20 and SK-BR-3 cells only expressed ICAM-1 or VCAM-1 after stimulation with TNF-alpha. Eosinophils constitutively bound to MDA-MB-435S cells, but not to BT-20 cells. Stimulation with TNF-alpha slightly enhanced eosinophil adhesion to MDA-MB-435S cells and dramatically increased adhesion to BT-20 cells. Greater than 80% of eosinophil adhesion to these cell lines was blocked with an anti-alpha4-integrin monoclonal antibody. Both MDA-MB-435S and BT-20 cells also released eosinophil activator(s). Supernatants from TNF-alpha-treated, but not control-treated, cell lines increased eosinophil adhesion to fibronectin and increased eosinophil transmigration across fibronectin-coated transwell plates. Enzyme-linked immunosorbent assays showed that TNF-alpha-stimulated breast carcinoma cells released the chemokine regulated on activation, T cell expressed and secreted (RANTES). Addition of an anti-RANTES antibody to breast carcinoma cell supernatants partially blocked eosinophil activation suggesting that RANTES in these supernatants was participating in eosinophil activation. These data show that TNF-alpha-stimulated breast carcinoma cells express mediators that can both bind and activate eosinophils, suggesting a mechanism for eosinophil localization to breast carcinoma sites.
Figures


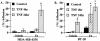
Similar articles
-
CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1.Ann Allergy Asthma Immunol. 2005 Feb;94(2):292-300. doi: 10.1016/S1081-1206(10)61311-7. Ann Allergy Asthma Immunol. 2005. PMID: 15765748
-
Differential regulation of eosinophil adhesion and transmigration by pulmonary microvascular endothelial cells.J Immunol. 1998 Jul 15;161(2):971-7. J Immunol. 1998. PMID: 9670977
-
A CD18/ICAM-1-dependent pathway mediates eosinophil adhesion to human bronchial epithelial cells.Am J Respir Cell Mol Biol. 1998 Sep;19(3):408-18. doi: 10.1165/ajrcmb.19.3.3179. Am J Respir Cell Mol Biol. 1998. PMID: 9730868
-
Regulatory mechanisms of eosinophil adhesion to and transmigration across endothelial cells by alpha4 and beta2 integrins.Int Arch Allergy Immunol. 1999;120 Suppl 1:24-6. doi: 10.1159/000053588. Int Arch Allergy Immunol. 1999. PMID: 10529598 Review.
-
Adhesion interactions involved in eosinophil migration through vascular endothelium.Ann N Y Acad Sci. 1996 Oct 31;796:124-37. doi: 10.1111/j.1749-6632.1996.tb32574.x. Ann N Y Acad Sci. 1996. PMID: 8906219 Review.
Cited by
-
Changes in the expression of CD106, osteogenic genes, and transcription factors involved in the osteogenic differentiation of human bone marrow mesenchymal stem cells.J Bone Miner Metab. 2008;26(4):312-20. doi: 10.1007/s00774-007-0842-0. Epub 2008 Jul 4. J Bone Miner Metab. 2008. PMID: 18600396
-
Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions.Breast Cancer Res. 2003;5(1):31-6. doi: 10.1186/bcr554. Epub 2002 Oct 28. Breast Cancer Res. 2003. PMID: 12559043 Free PMC article. Review.
-
Chemokines: key players in cancer progression and metastasis.Front Biosci (Schol Ed). 2011 Jun 1;3(4):1569-82. doi: 10.2741/246. Front Biosci (Schol Ed). 2011. PMID: 21622291 Free PMC article. Review.
-
Viral Vector-Based Melanoma Gene Therapy.Biomedicines. 2020 Mar 16;8(3):60. doi: 10.3390/biomedicines8030060. Biomedicines. 2020. PMID: 32187995 Free PMC article. Review.
-
Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3.J Exp Med. 2001 Dec 17;194(12):1699-709. doi: 10.1084/jem.194.12.1699. J Exp Med. 2001. PMID: 11748272 Free PMC article.
References
-
- Wardlaw AJ, Moqbel R, Kay AB: Eosinophils: biology and role in disease. Adv Immunol 1995, 60:151-266 - PubMed
-
- Samoszuk M, Ramzi E: IgE, Reed-Sternberg cells, and eosinophilia in Hodgkin’s disease. Leuk Lymphoma 1993, 9:315-319 - PubMed
-
- Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, Askin FB: The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg 1992, 106:27-33 - PubMed
-
- Samoszuk M: Eosinophils and human cancer. Histol Histopathol 1997, 12:807-812 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

