Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss
- PMID: 10880403
- PMCID: PMC1850215
- DOI: 10.1016/s0002-9440(10)64544-0
Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss
Erratum in
- Am J Pathol 2000 Oct;157(4):1413
Abstract
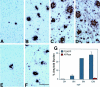
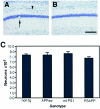
To analyze the relationship between the deposition of amyloid beta peptides (Abeta) and neuronal loss in transgenic models of Alzheimer's disease (AD), we examined the frontal neocortex (Fc) and CA1 portion of hippocampus (CA1) in PSAPP mice doubly expressing AD-associated mutant presenilin 1 (PS1) and Swedish-type mutant beta amyloid precursor protein (APPsw) by morphometry of Abeta burden and neuronal counts. Deposition of Abeta was detected as early as 3 months of age in the Fc and CA1 of PSAPP mice and progressed to cover 28.3% of the superior frontal cortex and 18.4% of CA1 at 12 months: approximately 20- (Fc) and approximately 40- (CA1) fold greater deposition than in APPsw mice. There was no significant difference in neuronal counts in either CA1 or the frontal cortex between nontransgenic (non-tg), PS1 transgenic, APPsw, and PSAPP mice at 3 to 12 months of age. In the PSAPP mice, there was disorganization of the neuronal architecture by compact amyloid plaques, and the average number of neurons was 8 to 10% fewer than the other groups (NS, P > 0.10) in CA1 and 2 to 20% fewer in frontal cortex (NS, P = 0.31). There was no loss of total synaptophysin immunoreactivity in the Fc or dentate gyrus molecular layer of the 12-month-old PSAPP mice. Thus, although co-expression of mutant PS1 with Swedish mutant betaAPP leads to marked cortical and limbic Abeta deposition in an age-dependent manner, it does not result in the dramatic neuronal loss in hippocampus and association cortex characteristic of AD.
Figures




Similar articles
-
Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice.J Neuropathol Exp Neurol. 2004 May;63(5):418-28. doi: 10.1093/jnen/63.5.418. J Neuropathol Exp Neurol. 2004. PMID: 15198121
-
Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes.Neurobiol Dis. 1999 Aug;6(4):231-44. doi: 10.1006/nbdi.1999.0243. Neurobiol Dis. 1999. PMID: 10448051
-
Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition.J Neurosci. 2002 May 1;22(9):3376-85. doi: 10.1523/JNEUROSCI.22-09-03376.2002. J Neurosci. 2002. PMID: 11978814 Free PMC article.
-
Metabolism of presenilin 1: influence of presenilin 1 on amyloid precursor protein processing.Neurobiol Aging. 1998 Jan-Feb;19(1 Suppl):S15-8. doi: 10.1016/s0197-4580(98)00026-8. Neurobiol Aging. 1998. PMID: 9562461 Review.
-
Progress toward valid transgenic mouse models for Alzheimer's disease.Neurobiol Aging. 1999 Mar-Apr;20(2):201-11. doi: 10.1016/s0197-4580(99)00042-1. Neurobiol Aging. 1999. PMID: 10537029 Review.
Cited by
-
Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production.J Neurosci. 2013 Jul 10;33(28):11606-17. doi: 10.1523/JNEUROSCI.0954-13.2013. J Neurosci. 2013. PMID: 23843529 Free PMC article.
-
High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner.Neurobiol Aging. 2019 Feb;74:121-134. doi: 10.1016/j.neurobiolaging.2018.10.018. Epub 2018 Oct 23. Neurobiol Aging. 2019. PMID: 30448612 Free PMC article.
-
FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice.J Clin Invest. 2003 Oct;112(8):1202-10. doi: 10.1172/JCI16618. J Clin Invest. 2003. PMID: 14561705 Free PMC article.
-
Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease.Am J Pathol. 2003 Dec;163(6):2155-64. doi: 10.1016/s0002-9440(10)63572-9. Am J Pathol. 2003. PMID: 14633589 Free PMC article.
-
Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer's disease.Brain Struct Funct. 2010 Mar;214(2-3):145-60. doi: 10.1007/s00429-010-0242-4. Epub 2010 Mar 7. Brain Struct Funct. 2010. PMID: 20213270 Free PMC article.
References
-
- Hyman BT, Trojanowski JQ: Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997, 56:1095-1097 - PubMed
-
- Suzuki N, Cheung TT, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG: An increased percentage of long amyloid β-protein is secreted by familial amyloid β-protein precursor (βAPP717) mutants. Science 1994, 264:1336-1340 - PubMed
-
- Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S: Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 1996, 383:710-713 - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS: Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron 1996, 17:1005-1013 - PubMed
-
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George-Hyslop P, Selkoe DJ : Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat Med 1997, 3:67–72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

