Salmonella maintains the integrity of its intracellular vacuole through the action of SifA
- PMID: 10880437
- PMCID: PMC313946
- DOI: 10.1093/emboj/19.13.3235
Salmonella maintains the integrity of its intracellular vacuole through the action of SifA
Erratum in
- EMBO J 2000 Aug 1;19(15):4191
Abstract
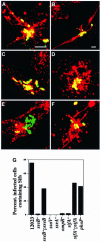
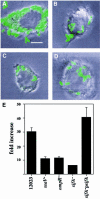
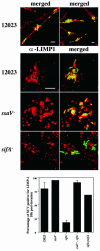
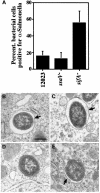
A method based on the Competitive Index was used to identify Salmonella typhimurium virulence gene interactions during systemic infections of mice. Analysis of mixed infections involving single and double mutant strains showed that OmpR, the type III secretion system of Salmonella pathogenicity island 2 (SPI-2) and SifA [required for the formation in epithelial cells of lysosomal glycoprotein (lgp)-containing structures, termed Sifs] are all involved in the same virulence function. sifA gene expression was induced after Salmonella entry into host cells and was dependent on the SPI-2 regulator ssrA. A sifA(-) mutant strain had a replication defect in macrophages, similar to that of SPI-2 and ompR(-) mutant strains. Whereas wild-type and SPI-2 mutant strains reside in vacuoles that progressively acquire lgps and the vacuolar ATPase, the majority of sifA(-) bacteria lost their vacuolar membrane and were released into the host cell cytosol. We propose that the wild-type strain, through the action of SPI-2 effectors (including SpiC), diverts the Salmonella-containing vacuole from the endocytic pathway, and subsequent recruitment and maintenance of vacuolar ATPase/lgp-containing membranes that enclose replicating bacteria is mediated by translocation of SifA.
Figures





References
-
- Beuzón C., Banks,G., Deiwick,J., Hensel,M. and Holden,D.W. (1999) pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol., 33, 806–816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

