Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice
- PMID: 10880440
- PMCID: PMC313956
- DOI: 10.1093/emboj/19.13.3272
Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice
Abstract
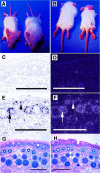
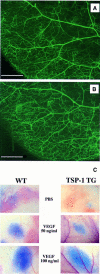
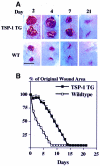
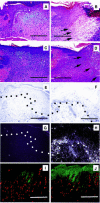
The function of the endogenous angiogenesis inhibitor thrombospondin-1 (TSP-1) in tissue repair has remained controversial. We established transgenic mice with targeted overexpression of TSP-1 in the skin, using a keratin 14 expression cassette. TSP-1 transgenic mice were healthy and fertile, and did not show any major abnormalities of normal skin vascularity, cutaneous vascular architecture, or microvascular permeability. However, healing of full-thickness skin wounds was greatly delayed in TSP-1 transgenic mice and was associated with reduced granulation tissue formation and highly diminished wound angiogenesis. Moreover, TSP-1 potently inhibited fibroblast migration in vivo and in vitro. These findings demonstrate that TSP-1 preferentially interfered with wound healing-associated angiogenesis, rather than with the angiogenesis associated with normal development and skin homeostasis, and suggest that therapeutic application of angiogenesis inhibitors might potentially be associated with impaired wound vascularization and tissue repair.
Figures



References
-
- Adams J.C. (1997) Thrombospondin-1. Int. J. Biochem. Cell Biol., 29, 861–865. - PubMed
-
- Ando Y. and Jensen,P. (1996) Protein kinase C mediates up-regulation of urokinase and its receptor in the migrating keratinocytes of wounded cultures, but urokinase is not required for movement across a substratum in vitro. J. Cell Physiol., 167, 500–511. - PubMed
-
- Arnold F. and West,D.C. (1991) Angiogenesis in wound healing. Pharmacol. Ther., 52, 407–422. - PubMed
-
- Beer H.-D., Longaker,M.T. and Werner,S. (1997) Reduced expression of PDGF and PDGF receptors during impaired wound healing. J. Invest. Dermatol., 109, 132–138. - PubMed
-
- Border W.A. and Noble,N.A. (1994) Transforming growth factor β in tissue fibrosis. N. Engl. J. Med., 331, 1286–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

