CD40 signaling in human dendritic cells is initiated within membrane rafts
- PMID: 10880443
- PMCID: PMC313954
- DOI: 10.1093/emboj/19.13.3304
CD40 signaling in human dendritic cells is initiated within membrane rafts
Abstract
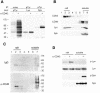
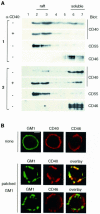
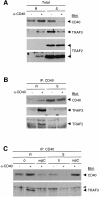
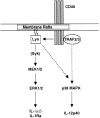
Despite CD40's role in stimulating dendritic cells (DCs) for efficient specific T-cell stimulation, its signal transduction components in DCs are still poorly documented. We show that CD40 receptors on human monocyte-derived DCs associate with sphingolipid- and cholesterol-rich plasma membrane microdomains, termed membrane rafts. Following engagement, CD40 utilizes membrane raft-associated Lyn Src family kinase, and possibly other raft-associated Src family kinases, to initiate tyrosine phosphorylation of intracellular substrates. CD40 engagement also leads to a membrane raft-restricted recruitment of tumor necrosis factor (TNF) receptor-associated factor (TRAF) 3 and, to a lesser extent, TRAF2, to CD40's cytoplasmic tail. Thus, the membrane raft structure plays an integral role in proximal events of CD40 signaling in DCs. We demonstrate that stimulation of Src family kinase within membrane rafts initiates a pathway implicating ERK activation, which leads to interleukin (IL)-1alpha/beta and IL-1Ra mRNA production and contributes to p38-dependent IL-12 mRNA production. These results provide the first evidence that membrane rafts play a critical role in initiation of CD40 signaling in DCs, and delineate the outcome of CD40-mediated pathways on cytokine production.
Figures


Similar articles
-
CD40 translocation to lipid rafts: signaling requirements and downstream biological events.Eur J Immunol. 2011 Aug;41(8):2358-67. doi: 10.1002/eji.201041143. Epub 2011 Jul 4. Eur J Immunol. 2011. PMID: 21567389
-
Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells.J Immunol. 1999 Dec 1;163(11):5786-95. J Immunol. 1999. PMID: 10570261
-
CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells.Am J Physiol Renal Physiol. 2002 Jun;282(6):F1020-33. doi: 10.1152/ajprenal.00291.2001. Am J Physiol Renal Physiol. 2002. PMID: 11997318
-
IgE receptor (Fc epsilon RI) and signal transduction.Eur Respir J Suppl. 1996 Aug;22:116s-118s. Eur Respir J Suppl. 1996. PMID: 8871055 Review.
-
Signaling through sphingolipid microdomains of the plasma membrane: the concept of signaling platform.Glycoconj J. 2000 Mar-Apr;17(3 -4):191-7. doi: 10.1023/a:1026585006064. Glycoconj J. 2000. PMID: 11201790 Review.
Cited by
-
L-Plastin Phosphorylation: Possible Regulation by a TNFR1 Signaling Cascade in Osteoclasts.Cells. 2021 Sep 15;10(9):2432. doi: 10.3390/cells10092432. Cells. 2021. PMID: 34572081 Free PMC article.
-
Roles of TRAF6 in CD40 signaling.Immunol Res. 2007;39(1-3):105-14. doi: 10.1007/s12026-007-0082-3. Immunol Res. 2007. PMID: 17917059 Review.
-
Pdro, a protein associated with late endosomes and lysosomes and implicated in cellular cholesterol homeostasis.PLoS One. 2010 Jun 8;5(6):e10977. doi: 10.1371/journal.pone.0010977. PLoS One. 2010. PMID: 20544018 Free PMC article.
-
Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry.J Virol. 2003 Sep;77(17):9542-52. doi: 10.1128/jvi.77.17.9542-9552.2003. J Virol. 2003. PMID: 12915568 Free PMC article.
-
Per a 10 protease activity modulates CD40 expression on dendritic cell surface by nuclear factor-kappaB pathway.Clin Exp Immunol. 2015 May;180(2):341-51. doi: 10.1111/cei.12569. Clin Exp Immunol. 2015. PMID: 25492061 Free PMC article. Clinical Trial.
References
-
- Aicher A., Shu,G.L., Magaletti,D., Mulvania,T., Pezzutto,A., Craxton,A. and Clark,E.A. (1999) Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J. Immunol., 163, 5786–5795. - PubMed
-
- Alessi D.R., Cuenda,A., Cohen,P., Dudley,D.T. and Saltiel,A.R. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem., 270, 27489–27494. - PubMed
-
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. - PubMed
-
- Banchereau J. and Steinman,R.M. (1998) Dendritic cells and the control of immunity. Nature, 392, 245–252. - PubMed
-
- Bennett S.R., Carbone,F.R., Karamalis,F., Flavell,R.A., Miller,J.F. and Heath,W.R. (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature, 393, 478–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

