The apoptotic signaling pathway activated by Toll-like receptor-2
- PMID: 10880445
- PMCID: PMC313930
- DOI: 10.1093/emboj/19.13.3325
The apoptotic signaling pathway activated by Toll-like receptor-2
Abstract
The innate immune system uses Toll family receptors to signal for the presence of microbes and initiate host defense. Bacterial lipoproteins (BLPs), which are expressed by all bacteria, are potent activators of Toll-like receptor-2 (TLR2). Here we show that the adaptor molecule, myeloid differentiation factor 88 (MyD88), mediates both apoptosis and nuclear factor-kappaB (NF-kappaB) activation by BLP-stimulated TLR2. Inhibition of the NF-kappaB pathway downstream of MyD88 potentiates apoptosis, indicating that these two pathways bifurcate at the level of MyD88. TLR2 signals for apoptosis through MyD88 via a pathway involving Fas-associated death domain protein (FADD) and caspase 8. Moreover, MyD88 binds FADD and is sufficient to induce apoptosis. These data indicate that TLR2 is a novel 'death receptor' that engages the apoptotic machinery without a conventional cytoplasmic death domain. Through TLR2, BLP induces the synthesis of the precursor of the pro-inflammatory cytokine interleukin-1beta (IL-1beta). Interestingly, BLP also activates caspase 1 through TLR2, resulting in proteolysis and secretion of mature IL-1beta. These results indicate that caspase activation is an innate immune response to microbial pathogens, culminating in apoptosis and cytokine production.
Figures

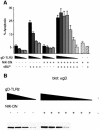


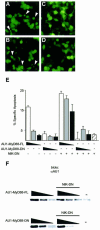

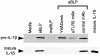
References
-
- Adachi O., Kawai,T., Takeda,K., Matsumoto,M., Tsutsui,H., Sakagami,M., Nakanishi,K. and Akira,S. (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity, 9, 143–150. - PubMed
-
- Aliprantis A.O., Yang,R.B., Mark,M.R., Suggett,S., Devaux,B., Radolf,J.D., Klimpel,G.R., Godowski,P. and Zychlinsky,A. (1999) Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science, 285, 736–739. - PubMed
-
- Ashkenazi A. and Dixit,V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. - PubMed
-
- Baeuerle P.A. (1998) IκB–NF-κB structures: at the interface of inflammation control. Cell, 95, 729–731. - PubMed
-
- Baeuerle P.A. and Baichwal,V.R. (1997) NF-κ B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol., 65, 111–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

