Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice
- PMID: 10880446
- PMCID: PMC313949
- DOI: 10.1093/emboj/19.13.3337
Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice
Abstract
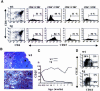
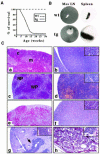
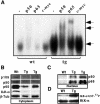
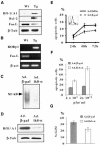
The multiplicity of Notch receptors raises the question of the contribution of specific isoforms to T-cell development. Notch3 is expressed in CD4(-)8(-) thymocytes and is down-regulated across the CD4(-)8(-) to CD4(+)8(+) transition, controlled by pre-T-cell receptor signaling. To determine the effects of Notch3 on thymocyte development, transgenic mice were generated, expressing lck promoter-driven intracellular Notch3. Thymuses of young transgenics showed an increased number of thymocytes, particularly late CD4(-)8(-) cells, a failure to down-regulate CD25 in post-CD4(-)8(-) subsets and sustained activity of NF-kappaB. Subsequently, aggressive multicentric T-cell lymphomas developed with high penetrance. Tumors sustained characteristics of immature thymocytes, including expression of CD25, pTalpha and activated NF-kappaB via IKKalpha-dependent degradation of IkappaBalpha and enhancement of NF-kappaB-dependent anti-apoptotic and proliferative pathways. Together, these data identify activated Notch3 as a link between signals leading to NF-kappaB activation and T-cell tumorigenesis. The phenotypes of pre-malignant thymocytes and of lymphomas indicate a novel and particular role for Notch3 in co-ordinating growth and differentiation of thymocytes, across the pre-T/T cell transition, consistent with the normal expression pattern of Notch3.
Figures




References
-
- Alesse E., Zazzeroni,F., Angelucci,A., Giannini,G., Di Marcotullio,L. and Gulino,A. (1998) The growth arrest and downregulation of c-myc transcription induced by ceramide are related events dependent on p21 induction, Rb underphosphorylation and E2F sequestering. Cell Death Differ., 5, 381–389. - PubMed
-
- Apelqvist A., Li,H., Sommer,L., Beatus,P., Anderson,D.J., Honjio,T., Hrabe de Angelis,M., Lendahl,U. and Edlund,H. (1999) Notch signalling controls pancreatic cell differentiation. Nature, 400, 877–881. - PubMed
-
- Artavanis-Tsakonas S., Rand,M.D. and Lake,R. (1999) Notch signaling: cell fate control and signal integration in development. Science, 284, 770–776. - PubMed
-
- Bakker T.R., Renno,T. and Jongeneel,C.V. (1999) Impaired fetal thymocyte development after efficient adenovirus-mediated inhibition of NF-κB activation. J. Immunol., 162, 3456–3462. - PubMed
-
- Barber D.F., Passoni,L., Wen,L., Geng,L. and Hayday,A.C. (1998) The expression in vivo of a second isoform of pTα: implications for the mechanism of pTα action. J. Immunol., 161, 11–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

