Isolation and characterization of Tn7 transposase gain-of-function mutants: a model for transposase activation
- PMID: 10880457
- PMCID: PMC313929
- DOI: 10.1093/emboj/19.13.3446
Isolation and characterization of Tn7 transposase gain-of-function mutants: a model for transposase activation
Abstract
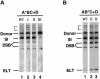
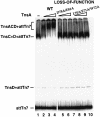
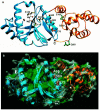
Tn7 transposition has been hypothesized to require a heteromeric transposase formed by two Tn7-encoded proteins, TnsA and TnsB, and accessory proteins that activate the transposase when they are associated with an appropriate target DNA. This study investigates the mechanism of Tn7 transposase activation by isolation and analysis of transposase gain-of-function mutants that are active in the absence of these accessory proteins. This work shows directly that TnsA and TnsB are essential and sufficient components of the Tn7 transposase and also provides insight into the signals that activate the transposase. We also describe a protein-protein interaction between TnsA and TnsC, a regulatory accessory protein, that is likely to be critical for transposase activation.
Figures

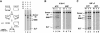



Similar articles
-
Direct interaction between the TnsA and TnsB subunits controls the heteromeric Tn7 transposase.Proc Natl Acad Sci U S A. 2013 May 28;110(22):E2038-45. doi: 10.1073/pnas.1305716110. Epub 2013 May 14. Proc Natl Acad Sci U S A. 2013. PMID: 23674682 Free PMC article.
-
Alternative interactions between the Tn7 transposase and the Tn7 target DNA binding protein regulate target immunity and transposition.EMBO J. 2003 Nov 3;22(21):5904-17. doi: 10.1093/emboj/cdg551. EMBO J. 2003. PMID: 14592987 Free PMC article.
-
The Tn7 transposition regulator TnsC interacts with the transposase subunit TnsB and target selector TnsD.Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):E2858-65. doi: 10.1073/pnas.1409869111. Epub 2014 Jun 30. Proc Natl Acad Sci U S A. 2014. PMID: 24982178 Free PMC article.
-
Tn7.Microbiol Spectr. 2014 Oct;2(5). doi: 10.1128/microbiolspec.MDNA3-0010-2014. Microbiol Spectr. 2014. PMID: 26104363 Review.
-
Heteromeric transposase elements: generators of genomic islands across diverse bacteria.Mol Microbiol. 2014 Sep;93(6):1084-92. doi: 10.1111/mmi.12740. Epub 2014 Aug 19. Mol Microbiol. 2014. PMID: 25091064 Review.
Cited by
-
Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7.Mob DNA. 2010 Jul 23;1(1):18. doi: 10.1186/1759-8753-1-18. Mob DNA. 2010. PMID: 20653944 Free PMC article.
-
Direct interaction between the TnsA and TnsB subunits controls the heteromeric Tn7 transposase.Proc Natl Acad Sci U S A. 2013 May 28;110(22):E2038-45. doi: 10.1073/pnas.1305716110. Epub 2013 May 14. Proc Natl Acad Sci U S A. 2013. PMID: 23674682 Free PMC article.
-
Tn7 recognizes transposition target structures associated with DNA replication using the DNA-binding protein TnsE.Genes Dev. 2001 Mar 15;15(6):737-47. doi: 10.1101/gad.870201. Genes Dev. 2001. PMID: 11274058 Free PMC article.
-
Tn7 elements: engendering diversity from chromosomes to episomes.Plasmid. 2009 Jan;61(1):1-14. doi: 10.1016/j.plasmid.2008.09.008. Epub 2008 Nov 1. Plasmid. 2009. PMID: 18951916 Free PMC article. Review.
-
The carboxy-terminal portion of TnsC activates the Tn7 transposase through a specific interaction with TnsA.EMBO J. 2004 Aug 4;23(15):2972-81. doi: 10.1038/sj.emboj.7600311. Epub 2004 Jul 15. EMBO J. 2004. PMID: 15257292 Free PMC article.
References
-
- Arciszewska L.K., McKown,R.L. and Craig,N.L. (1991) Purification of TnsB, a transposition protein that binds to the ends of Tn7. J. Biol. Chem., 266, 21736–21744. - PubMed
-
- Bainton R., Gamas,P. and Craig,N.L. (1991) Tn7 transposition in vitro proceeds through an excised transposon intermediate generated by staggered breaks in DNA. Cell, 65, 805–816. - PubMed
-
- Bainton R.J., Kubo,K.M., Feng,J.-N. and Craig,N.L. (1993) Tn7 transposition: target DNA recognition is mediated by multiple Tn7-encoded proteins in a purified in vitro system. Cell, 72, 931–943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

