HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function
- PMID: 10880527
- PMCID: PMC1887711
- DOI: 10.1084/jem.192.1.63
HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function
Abstract
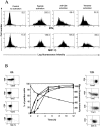
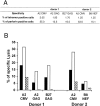
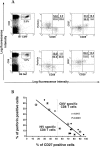
The use of peptide-human histocompatibility leukocyte antigen (HLA) class I tetrameric complexes to identify antigen-specific CD8(+) T cells has provided a major development in our understanding of their role in controlling viral infections. However, questions remain about the exact function of these cells, particularly in HIV infection. Virus-specific cytotoxic T lymphocytes exert much of their activity by secreting soluble factors such as cytokines and chemokines. We describe here a method that combines the use of tetramers and intracellular staining to examine the functional heterogeneity of antigen-specific CD8(+) T cells ex vivo. After stimulation by specific peptide antigen, secretion of interferon (IFN)-gamma, tumor necrosis factor (TNF)-alpha, macrophage inflammatory protein (MIP)-1beta, and perforin is analyzed by FACS((R)) within the tetramer-positive population in peripheral blood. Using this method, we have assessed the functional phenotype of HIV-specific CD8(+) T cells compared with cytomegalovirus (CMV)-specific CD8(+) T cells in HIV chronic infection. We show that the majority of circulating CD8(+) T cells specific for CMV and HIV antigens are functionally active with regards to the secretion of antiviral cytokines in response to antigen, although a subset of tetramer-staining cells was identified that secretes IFN-gamma and MIP-1beta but not TNF-alpha. However, a striking finding is that HIV-specific CD8(+) T cells express significantly lower levels of perforin than CMV-specific CD8(+) T cells. This lack of perforin is linked with persistent CD27 expression on HIV-specific cells, suggesting impaired maturation, and specific lysis ex vivo is lower for HIV-specific compared with CMV-specific cells from the same donor. Thus, HIV-specific CD8(+) T cells are impaired in cytolytic activity.
Figures




References
-
- Carmichael A., Jin X., Sissons P., Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)–specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infectiondifferential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 1993;177:249–256. - PMC - PubMed
-
- Kalams S.A., Johnson R.P., Trocha A.K., Dynan M.J., Ngo S., D'Aquila R.T., Kurnick J.T., Walker B.D. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope–specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J. Exp. Med. 1994;179:1261–1271. - PMC - PubMed
-
- Kern F., Surel I.P., Brock C., Freistedt B., Radtke H., Scheffold A., Blasczyk R., Reinke P., Schneider-Mergener J., Radbruch A. T-cell epitope mapping by flow cytometry. Nat. Med. 1998;4:975–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

