Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells
- PMID: 10880532
- PMCID: PMC1887703
- DOI: 10.1084/jem.192.1.117
Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells
Abstract
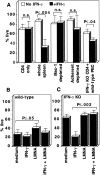
In Mycobacterium bovis Bacille Calmette-Guérin (BCG)-infected wild-type mice, there was a large expansion of an activated (CD44(hi)) splenic CD4 T cell population followed by a rapid contraction of this population to normal numbers. Contraction of the activated CD4 T cell population in wild-type mice was associated with increased apoptosis of activated CD4 T cells. In BCG-infected interferon (IFN)-gamma knockout (KO) mice, the activated CD4 T cell population did not undergo apoptosis. These mice accumulated large numbers of CD4(+)CD44(hi) T cells that were responsive to mycobacterial antigens. Addition of IFN-gamma to cultured splenocytes from BCG-infected IFN-gamma KO mice induced apoptosis of activated CD4 T cells. IFN-gamma-mediated apoptosis was abolished by depleting adherent cells or Mac-1(+) spleen cells or by inhibiting nitric oxide synthase. Thus, IFN-gamma is essential to a regulatory mechanism that eliminates activated CD4 T cells and maintains CD4 T cell homeostasis during an immune response.
Figures



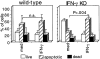
References
-
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cellsa reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. - PubMed
-
- Van Parijs L., Abbas A.K. Homeostasis and self-tolerance in the immune systemturning lymphocytes off. Science. 1998;280:243–248. - PubMed
-
- Chambers C.A., Krummel M.F., Boitel B., Hurwitz A., Sullivan T.J., Fournier S., Cassell D., Brunner M., Allison J.P. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol. Rev. 1996;153:27–46. - PubMed
-
- Van Parijs L., Peterson D.A., Abbas A.K. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity. 1998;8:265–274. - PubMed
-
- Bachmann M.F., Waterhouse P., Speiser D.E., McKall-Faienza K., Mak T.W., Ohashi P.S. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J. Immunol. 1998;160:95–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

