The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling
- PMID: 10880563
- PMCID: PMC26957
- DOI: 10.1073/pnas.140087397
The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling
Abstract
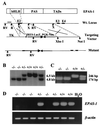
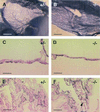
We have studied the role of the basic helix-loop-helix-PAS transcription factor EPAS-1/hypoxia-inducible factor 2alpha in vascular development by gene targeting. In ICR/129 Sv outbred background, more than half of the mutants displayed varying degrees of vascular disorganization, typically in the yolk sac, and died in utero between embryonic day (E)9.5 and E13.5. In mutant embryos directly derived from EPAS-1(-/-) embryonic stem cells (hence in 129 Sv background), all embryos developed severe vascular defects both in the yolk sac and embryo proper and died between E9.5 and E12.5. Normal blood vessels were formed by vasculogenesis but they either fused improperly or failed to assemble into larger vessels later during development. Our results suggest that EPAS-1 plays an important role at postvasculogenesis stages and is required for the remodeling of the primary vascular network into a mature hierarchy pattern.
Figures




Similar articles
-
Extra-embryonic vasculature development is regulated by the transcription factor HAND1.Development. 2004 May;131(9):2195-204. doi: 10.1242/dev.01091. Epub 2004 Apr 8. Development. 2004. PMID: 15073150
-
The bHLH/PAS factor MOP3 does not participate in hypoxia responses.Biochem Biophys Res Commun. 2002 Feb 1;290(4):1228-36. doi: 10.1006/bbrc.2001.6309. Biochem Biophys Res Commun. 2002. PMID: 11811994
-
Targeted replacement of hypoxia-inducible factor-1alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth.Cancer Res. 2005 Mar 15;65(6):2277-86. doi: 10.1158/0008-5472.CAN-04-3246. Cancer Res. 2005. PMID: 15781641
-
Role of hypoxia-inducible factor-2alpha in endothelial development and hematopoiesis.Methods Enzymol. 2007;435:199-218. doi: 10.1016/S0076-6879(07)35011-8. Methods Enzymol. 2007. PMID: 17998056 Review.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
-
EPAS1 Is Required for Spermatogenesis in the Postnatal Mouse Testis.Biol Reprod. 2010 Jun;82(6):1227-36. doi: 10.1095/biolreprod.109.079202. Epub 2010 Feb 24. Biol Reprod. 2010. PMID: 20181618 Free PMC article.
-
Improved vascular survival and growth in the mouse model of hindlimb ischemia by a remote signaling mechanism.Am J Pathol. 2014 Mar;184(3):686-96. doi: 10.1016/j.ajpath.2013.11.032. Epub 2014 Jan 17. Am J Pathol. 2014. PMID: 24440788 Free PMC article.
-
Translating the Hypoxic Response-the Role of HIF Protein Translation in the Cellular Response to Low Oxygen.Cells. 2019 Feb 1;8(2):114. doi: 10.3390/cells8020114. Cells. 2019. PMID: 30717305 Free PMC article. Review.
-
In vitro study of enhanced osteogenesis induced by HIF-1α-transduced bone marrow stem cells.Cell Prolif. 2011 Jun;44(3):234-43. doi: 10.1111/j.1365-2184.2011.00747.x. Cell Prolif. 2011. PMID: 21535264 Free PMC article.
-
Hypoxic signaling during tissue repair and regenerative medicine.Int J Mol Sci. 2014 Oct 31;15(11):19791-815. doi: 10.3390/ijms151119791. Int J Mol Sci. 2014. PMID: 25365172 Free PMC article. Review.
References
-
- Leung D W, Cachianes G, Kuang W J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. - PubMed
-
- Senger D R, Connolly D T, Van de Water L, Feder J, Dvorak H F. Cancer Res. 1990;50:1774–1778. - PubMed
-
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Oncogene. 1990;5:519–524. - PubMed
-
- de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. Science. 1992;255:989–991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

