Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme
- PMID: 10880574
- PMCID: PMC26981
- DOI: 10.1073/pnas.150149097
Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme
Abstract
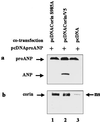
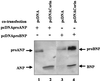
Atrial natriuretic peptide (ANP) is a cardiac hormone essential for the regulation of blood pressure. In cardiac myocytes, ANP is synthesized as a precursor, pro-ANP, that is converted to biologically active ANP by an unknown membrane-associated protease. Recently, we cloned a transmembrane serine protease, corin, that is highly expressed in the heart. In this study, we examine effects of corin on pro-ANP processing. Our results show that recombinant human corin converts pro-ANP to ANP and that the cleavage in pro-ANP by corin is highly sequence specific. Our findings suggest that corin is the long-sought pro-ANP-converting enzyme and that the corin-mediated pro-ANP activation may play a role in regulating blood pressure.
Figures


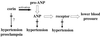
Similar articles
-
Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes.J Biol Chem. 2002 May 10;277(19):16900-5. doi: 10.1074/jbc.M201503200. Epub 2002 Mar 7. J Biol Chem. 2002. PMID: 11884416
-
Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms.J Mol Cell Cardiol. 2008 Jan;44(1):131-42. doi: 10.1016/j.yjmcc.2007.10.002. Epub 2007 Oct 11. J Mol Cell Cardiol. 2008. PMID: 17996891
-
Corin-mediated processing of pro-atrial natriuretic peptide in human small cell lung cancer cells.Cancer Res. 2003 Dec 1;63(23):8318-22. Cancer Res. 2003. PMID: 14678991
-
Function and regulation of corin in physiology and disease.Biochem Soc Trans. 2020 Oct 30;48(5):1905-1916. doi: 10.1042/BST20190760. Biochem Soc Trans. 2020. PMID: 33125488 Review.
-
The serine protease corin in cardiovascular biology and disease.Front Biosci. 2007 May 1;12:4179-90. doi: 10.2741/2379. Front Biosci. 2007. PMID: 17485366 Review.
Cited by
-
Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies.Eur Heart J. 2015 Mar 21;36(12):715-23. doi: 10.1093/eurheartj/ehu484. Epub 2015 Feb 10. Eur Heart J. 2015. PMID: 25670819 Free PMC article. Review.
-
Association of Preablation Plasma Corin Levels With Atrial Fibrillation Recurrence After Catheter Ablation: A Prospective Observational Study.J Am Heart Assoc. 2024 Jan 16;13(2):e031928. doi: 10.1161/JAHA.123.031928. Epub 2024 Jan 12. J Am Heart Assoc. 2024. PMID: 38214265 Free PMC article.
-
Atrial natriuretic peptide in cardiovascular biology and disease (NPPA).Gene. 2015 Sep 10;569(1):1-6. doi: 10.1016/j.gene.2015.06.029. Epub 2015 Jun 12. Gene. 2015. PMID: 26074089 Free PMC article. Review.
-
Host Peptidic Hormones Affecting Bacterial Biofilm Formation and Virulence.J Innate Immun. 2019;11(3):227-241. doi: 10.1159/000493926. Epub 2018 Nov 5. J Innate Immun. 2019. PMID: 30396172 Free PMC article. Review.
-
Distinct roles of N-glycosylation at different sites of corin in cell membrane targeting and ectodomain shedding.J Biol Chem. 2015 Jan 16;290(3):1654-63. doi: 10.1074/jbc.M114.606442. Epub 2014 Dec 1. J Biol Chem. 2015. PMID: 25451932 Free PMC article.
References
-
- Oparil S. In: Cecil Textbook of Medicine. Bennett J C, Plum F, editors. Philadelphia: Saunders; 1996. pp. 256–271.
-
- de Bold A J, Borenstein H B, Veress A T, Sonnenberg H. Life Sci. 1981;28:89–94. - PubMed
-
- Inagami T. J Biol Chem. 1989;264:3043–3046. - PubMed
-
- Brenner B M, Ballermann B J, Gunning M E, Zeidel M L. Physiol Rev. 1990;70:665–699. - PubMed
-
- Rosenzweig A, Seidman C E. Annu Rev Biochem. 1991;60:229–255. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

