Distribution and development of nicotinic acetylcholine receptor subtypes in the optic tectum of Rana pipiens
- PMID: 10880991
- PMCID: PMC2265082
- DOI: 10.1002/1096-9861(20000807)423:4<603::aid-cne6>3.0.co;2-f
Distribution and development of nicotinic acetylcholine receptor subtypes in the optic tectum of Rana pipiens
Abstract
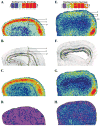
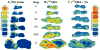
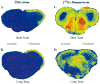
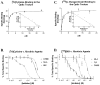
Acetylcholine allows the elicitation of visually evoked behaviors mediated by the frog optic tectum, but the mechanisms behind its effects are unknown. Although nicotinic acetylcholine receptors (nAChRs) exist in the tectum, their subtype has not been assessed. By using quantitative autoradiography, we examined the binding of [(3)H]cytisine and [(125)I]alpha-bungarotoxin in the laminated tectum. In mammalian systems, these radioligands bind with high affinity to alpha4 nAChR subunits and alpha7 nAChR subunits, respectively. [(3)H]Cytisine demonstrated high specific binding in adult frogs in retinorecipient layer 9, intermediate densities in layer 8, and low binding in layers 1-7 of the tectum. [(3)H]Cytisine binding was significantly higher in the tecta of adults than in those of tadpoles. Lesioning the optic nerve for 6 weeks decreased [(3)H]cytisine binding in layers 8/9 by 70+/-1%, whereas 6-month lesions decreased binding by 76+/-3%. Specific binding of [(125)I]alpha-bungarotoxin in adults was present only at intermediate levels in tectal layers 8 and 9, and undetectable in the deeper tectal layers. However, the nucleus isthmi, a midbrain structure reciprocally connected to the tectum, exhibited high levels of binding. There were no significant differences in tectal [(125)I]alpha-bungarotoxin binding between tadpoles and adults. Six-week lesions of the optic nerve decreased tectal [(125)I]alpha-bungarotoxin binding by 33+/-10%, but 6-month lesions had no effect. The pharmacokinetic characteristics of [(3)H]cytisine and [(125)I]alpha-bungarotoxin binding in the frog brain were similar to those demonstrated in several mammalian species. These results indicate that [(3)H]cytisine and [(125)I]alpha-bungarotoxin identify distinct nAChR subtypes in the tectum that likely contain non-alpha7 and alpha7 subunits, respectively. The majority of non-alpha7 receptors are likely associated with retinal ganglion cell terminals, whereas alpha7-containing receptors appear to have a different localization.
Copyright 2000 Wiley-Liss, Inc.
Figures

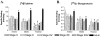


Similar articles
-
Pharmacology, distribution and development of muscarinic acetylcholine receptor subtypes in the optic tectum of Rana pipiens.Neuroscience. 2001;104(1):161-79. doi: 10.1016/s0306-4522(01)00048-3. Neuroscience. 2001. PMID: 11311540 Free PMC article.
-
Deletion of the alpha7, beta2, or beta4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine.Mol Pharmacol. 2006 Sep;70(3):947-59. doi: 10.1124/mol.106.025338. Epub 2006 May 25. Mol Pharmacol. 2006. PMID: 16728647
-
Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta.J Comp Neurol. 2003 Jun 16;461(1):49-60. doi: 10.1002/cne.10659. J Comp Neurol. 2003. PMID: 12722104
-
Comparative laminar distribution of various autoradiographic cholinergic markers in adult rat main olfactory bulb.J Chem Neuroanat. 1995 Aug;9(2):99-112. doi: 10.1016/0891-0618(95)00070-n. J Chem Neuroanat. 1995. PMID: 8561953
-
Effects of choline and other nicotinic agonists on the tectum of juvenile and adult Xenopus frogs: a patch-clamp study.Neuroscience. 1999;91(2):753-69. doi: 10.1016/s0306-4522(98)00625-3. Neuroscience. 1999. PMID: 10366031
Cited by
-
Bidirectional modulation of visual plasticity by cholinergic receptor subtypes in the frog optic tectum.Eur J Neurosci. 2003 Mar;17(6):1253-65. doi: 10.1046/j.1460-9568.2003.02557.x. Eur J Neurosci. 2003. PMID: 12670313 Free PMC article.
-
The effects of nicotinic and muscarinic receptor activation on patch-clamped cells in the optic tectum of Rana pipiens.Neuroscience. 2003;118(1):135-44. doi: 10.1016/s0306-4522(02)00768-6. Neuroscience. 2003. PMID: 12676145 Free PMC article.
-
Activity-dependent regulation of substance P expression and topographic map maintenance by a cholinergic pathway.J Neurosci. 2000 Jul 15;20(14):5346-57. doi: 10.1523/JNEUROSCI.20-14-05346.2000. J Neurosci. 2000. PMID: 10884319 Free PMC article.
-
Pharmacology, distribution and development of muscarinic acetylcholine receptor subtypes in the optic tectum of Rana pipiens.Neuroscience. 2001;104(1):161-79. doi: 10.1016/s0306-4522(01)00048-3. Neuroscience. 2001. PMID: 11311540 Free PMC article.
References
-
- Anand R, Peng X, Ballesta JJ, Lindstrom J. Pharmacological characterization of alpha-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of alpha 7 and alpha 8 subunit-containing subtypes. Mol Pharmacol. 1993;44:1046–1050. - PubMed
-
- Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. - PubMed
-
- Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S. alpha-bungarotoxin binding sites in rat hippocampal and cortical cultures: initial characterisation, colocalisation with alpha 7 subunits and up-regulation by chronic nicotine treatment. Brain Res. 1995;672:228–236. - PubMed
-
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

