Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals
- PMID: 10882397
- PMCID: PMC1572158
- DOI: 10.1038/sj.bjp.0703397
Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals
Abstract
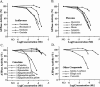
Mitochondrial proton F0F1-ATPase/ATP synthase synthesizes ATP during oxidative phosphorylation. In this study, we examined the effects of several groups of polyphenolic phytochemicals on the activity of the enzyme. Resveratrol, a stilbene phytoalexin that is present in grapes and red wine, concentration-dependently inhibited the enzymatic activity of both rat brain and liver F0F1-ATPase/ATP synthase (IC(50) of 12 - 28 microM). Screening of other polyphenolic phytochemicals using rat brain F0F1-ATPase activity resulted in the following ranking potency (IC(50) in parenthesis): piceatannol (8 microM)>resveratrol (19 microM)=(-)epigallocatechin gallate (17 microM)>(-)epicatechin gallate, curcumin (45 microM)>genistein=biochanin A=quercetin=kaempferol=morin (55 - 65 microM)>phloretin=apigenin=daidzein (approx. 100 microM). Genistin, quercitrin, phloridzin, (+)catechin, (+)epicatechin, (-)epicatechin and (-)epigallocatechin had little effect at similar concentrations. Tannic acid, theaflavins (tea extract) and grape seed proanthocyanidin extract (GSPE) had IC(50) values of 5, 20 and 30 microg ml(-1), respectively. Several monophenolic antioxidants and non-phenolic compounds were ineffective at concentrations of 210 microM or higher. The inhibition of F0F1-ATPase by resveratrol and genistein was non-competitive in nature. The effects of polyphenolic phytochemicals were additive. Both resveratrol and genistein had little effect on the Na(+)/K(+)-ATPase activity of porcine cerebral cortex, whereas quercetin had similar inhibitory potency as for F0F1-ATPase. In conclusion, the ATP synthase is a target for dietary phytochemicals. This pharmacological property of these phytochemicals should be included in the examination of their health benefits as well as potential cytotoxicity.
Figures




References
-
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S.-I., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. - PubMed
-
- ANTIPENKO A.Y., SPIELMAN A.I., KIRCHBERGER M.A. Interactions of 6-gingerol and ellagic acid with the cardiac sarcoplasmic reticulum Ca2+-ATPase. J. Pharmacol. Exp. Ther. 1999;290:227–234. - PubMed
-
- BARNES S., PETERSON T.G. Biochemical targets of the isoflavone genistein in tumor cell lines. Proc. Soc. Exp. Biol. Med. 1995;208:103–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

