Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2
- PMID: 10884417
- PMCID: PMC16637
- DOI: 10.1073/pnas.97.14.7871
Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2
Abstract
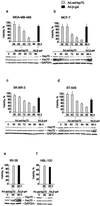
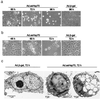
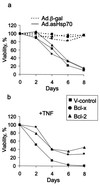
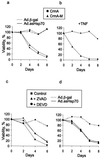
Heat shock protein 70 is an antiapoptotic chaperone protein highly expressed in human breast tumors and tumor cell lines. Here, we demonstrate that the mere inhibition of its synthesis by adenoviral transfer or classical transfection of antisense Hsp70 cDNA (asHsp70) results in massive death of human breast cancer cells (MDA-MB-468, MCF-7, BT-549, and SK-BR-3), whereas the survival of nontumorigenic breast epithelial cells (HBL-100) or fibroblasts (WI-38) is not affected. Despite the apoptotic morphology as judged by electron microscopy, the asHsp70-induced death was independent of known caspases and the p53 tumor suppressor protein. Furthermore, Bcl-2 and Bcl-X(L), which protect tumor cells from most forms of apoptosis, failed to rescue breast cancer cells from asHsp70-induced death. These results show that tumorigenic breast cancer cells depend on the constitutive high expression of Hsp70 to suppress a transformation-associated death program. Neutralization of Hsp70 may open new possibilities for treatment of cancers that have acquired resistance to therapies activating the classical apoptosis pathway.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

