Babesiosis
- PMID: 10885987
- PMCID: PMC88943
- DOI: 10.1128/CMR.13.3.451
Babesiosis
Abstract
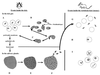
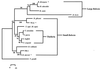
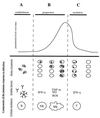
Babesiosis is an emerging, tick-transmitted, zoonotic disease caused by hematotropic parasites of the genus Babesia. Babesial parasites (and those of the closely related genus Theileria) are some of the most ubiquitous and widespread blood parasites in the world, second only to the trypanosomes, and consequently have considerable worldwide economic, medical, and veterinary impact. The parasites are intraerythrocytic and are commonly called piroplasms due to the pear-shaped forms found within infected red blood cells. The piroplasms are transmitted by ixodid ticks and are capable of infecting a wide variety of vertebrate hosts which are competent in maintaining the transmission cycle. Studies involving animal hosts other than humans have contributed significantly to our understanding of the disease process, including possible pathogenic mechanisms of the parasite and immunological responses of the host. To date, there are several species of Babesia that can infect humans, Babesia microti being the most prevalent. Infections with Babesia species generally follow regional distributions; cases in the United States are caused primarily by B. microti, whereas cases in Europe are usually caused by Babesia divergens. The spectrum of disease manifestation is broad, ranging from a silent infection to a fulminant, malaria-like disease, resulting in severe hemolysis and occasionally in death. Recent advances have resulted in the development of several diagnostic tests which have increased the level of sensitivity in detection, thereby facilitating diagnosis, expediting appropriate patient management, and resulting in a more accurate epidemiological description.
Figures

References
-
- Abdalla H S, Hussein H S, Kreier J P. Babesia rodhaini: passive protection of mice with immune serum. Tropenmed Parasitol. 1978;29:295–306. - PubMed
-
- Adachi K, Kawano H, Makimura S. Suppressed antibody response to sheep erythrocytes in experimentally Babesia rodhaini-infected mice. J Vet Med Sci. 1993;55:189–190. - PubMed
-
- Anderson A E, Cassaday P B, Healy G R. Babesiosis in man: sixth documented case. Am J Clin Pathol. 1974;62:612–618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

