Neutrophil lactoferrin release induced by IgA immune complexes differed from that induced by cross-linking of fcalpha receptors (FcalphaR) with a monoclonal antibody, MIP8a
- PMID: 10886246
- PMCID: PMC1905661
- DOI: 10.1046/j.1365-2249.2000.01254.x
Neutrophil lactoferrin release induced by IgA immune complexes differed from that induced by cross-linking of fcalpha receptors (FcalphaR) with a monoclonal antibody, MIP8a
Abstract
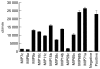
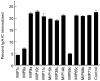
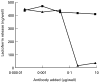
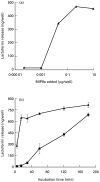
The human IgA Fc receptor (FcalphaR, CD89) plays an important role in host defence against invading pathogens. To study the properties of the receptor, 12 MoAbs, namely, MIP7c, MIP8a, MIP9a, MIP10c, MIP11c, MIP14b, MIP15b, MIP38c, MIP59c, MIP65c, MIP68b and MIP71a, were generated. The inhibitory effects of the antibodies on FcalphaR functions were tested. Three of the antibodies, MIP7c, MIP8a and MIP59c, were able to block up to 90% of soluble FcalphaR binding to IgA-coated beads and 70-80% of neutrophil phagocytosis of IgA immune complexes (IC). MIP8a could also inhibit IgA IC-induced neutrophil lactoferrin release, while cross-linking of FcalphaR with MIP8a and anti-mouse IgG could elicit neutrophil lactoferrin release. However, IgA IC-induced lactoferrin release required both extracellular calcium and magnesium, whereas MIP8a-induced release did not require extracellular magnesium and only partially required extracellular calcium. In addition, the time course of IgA IC-induced lactoferrin release was slow. Lactoferrin was not detectable if the incubation time was less than 0.5 h. In contrast, MIP8a-induced lactoferrin release was fast. Lactoferrin could be detected within 5 min of incubation. Therefore, neutrophil lactoferrin release induced by IgA IC differed from that induced by cross-linking of FcalphaR with MIP8a.
Figures


Similar articles
-
Neutrophil lactoferrin release induced by IgA immune complexes can be mediated either by Fc alpha receptors or by complement receptors through different pathways.J Immunol. 1996 Apr 1;156(7):2599-606. J Immunol. 1996. PMID: 8786325
-
Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human fcalpha receptor (FcalphaR) CD89.J Biol Chem. 1999 Aug 13;274(33):23508-14. doi: 10.1074/jbc.274.33.23508. J Biol Chem. 1999. PMID: 10438530
-
Deglycosylation of FcalphaR at N58 increases its binding to IgA.Glycobiology. 2010 Jul;20(7):905-15. doi: 10.1093/glycob/cwq048. Epub 2010 Apr 8. Glycobiology. 2010. PMID: 20378933
-
IgA Fc receptors in cattle and horses.Vet Immunol Immunopathol. 2005 Oct 18;108(1-2):139-43. doi: 10.1016/j.vetimm.2005.07.008. Vet Immunol Immunopathol. 2005. PMID: 16098605 Review.
-
The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review.
Cited by
-
Anti-CD20 IgA can protect mice against lymphoma development: evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells.Haematologica. 2012 Nov;97(11):1686-94. doi: 10.3324/haematol.2011.061408. Epub 2012 Jun 11. Haematologica. 2012. PMID: 22689689 Free PMC article.
-
Serum Soluble CD89-IgA Complexes Are Elevated in IgA Nephropathy without Immunosuppressant History.Dis Markers. 2020 Jan 16;2020:8393075. doi: 10.1155/2020/8393075. eCollection 2020. Dis Markers. 2020. PMID: 32076466 Free PMC article.
-
The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-alpha treatment in children with Henoch-Schönlein purpura.Clin Exp Immunol. 2002 Nov;130(2):352-7. doi: 10.1046/j.1365-2249.2002.01964.x. Clin Exp Immunol. 2002. PMID: 12390327 Free PMC article.
-
Pentraxins and IgA share a binding hot-spot on FcαRI.Protein Sci. 2014 Apr;23(4):378-86. doi: 10.1002/pro.2419. Epub 2014 Jan 28. Protein Sci. 2014. PMID: 24407959 Free PMC article.
-
Ectodomain shedding of Fcalpha receptor is mediated by ADAM10 and ADAM17.Immunology. 2010 May;130(1):83-91. doi: 10.1111/j.1365-2567.2009.03215.x. Epub 2010 Jan 6. Immunology. 2010. PMID: 20059578 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

