Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2
- PMID: 10887156
- PMCID: PMC316733
Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2
Abstract
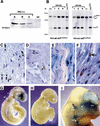
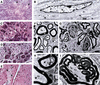
Hemizygosity for the NF2 gene in humans causes a syndromic susceptibility to schwannoma development. However, Nf2 hemizygous mice do not develop schwannomas but mainly osteosarcomas. In the tumors of both species, the second Nf2 allele is inactivated. We report that conditional homozygous Nf2 knockout mice with Cre-mediated excision of Nf2 exon 2 in Schwann cells showed characteristics of neurofibromatosis type 2. These included schwannomas, Schwann cell hyperplasia, cataract, and osseous metaplasia. Thus, the tumor suppressor function of Nf2, here revealed in murine Schwann cells, was concealed in hemizygous Nf2 mice because of insufficient rate of second allele inactivation in this cell compartment. The finding of this conserved function documents the relevance of the present approach to model the human disease.
Figures



References
-
- Bolande R. The neurochristopathies. A unifying concept of disease arising in neural crest maldevelopment. Hum Pathol. 1974;5:409–420. - PubMed
-
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development. 1993;117:409–429. - PubMed
-
- Deguen B, Merel P, Goutebroze L, Giovannini M, Reggio H, Arpin M, Thomas G. Impaired interaction of naturally occurring mutant NF2 protein with actin-based cytoskeleton and membrane. Hum Mol Genet. 1998;7:217–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
