Effects of palmitoylation of replicase protein nsP1 on alphavirus infection
- PMID: 10888610
- PMCID: PMC112188
- DOI: 10.1128/jvi.74.15.6725-6733.2000
Effects of palmitoylation of replicase protein nsP1 on alphavirus infection
Abstract
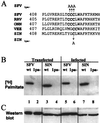
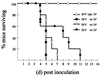
The membrane-associated alphavirus RNA replication complex contains four virus-encoded subunits, the nonstructural proteins nsP1 to nsP4. Semliki Forest virus (SFV) nsP1 is hydrophobically modified by palmitoylation of cysteines 418 to 420. Here we show that Sindbis virus nsP1 is also palmitoylated on the same site (cysteine 420). When mutations preventing nsP1 palmitoylation were introduced into the genomes of these two alphaviruses, the mutant viruses remained viable and replicated to high titers, although their growth was slightly delayed. The subcellular distribution of palmitoylation-defective nsP1 was altered in the mutant: it no longer localized to filopodial extensions, and a fraction of it was soluble. The ultrastructure of the alphavirus replication sites appeared normal, and the localization of the other nonstructural proteins was unaltered in the mutants. In both wild-type- and mutant-virus-infected cells, SFV nsP3 and nsP4 could be extracted from membranes only by alkaline solutions whereas the nsP2-membrane association was looser. Thus, the membrane binding properties of the alphavirus RNA replication complex were not determined by the palmitoylation of nsP1. The nsP1 palmitoylation-defective alphaviruses produced normal plaques in several cell types, but failed to give rise to plaques in HeLa cells, although they induced normal apoptosis of these cells. The SFV mutant was apathogenic in mice: it caused blood viremia, but no infectious virus was detected in the brain.
Figures



References
-
- Atkins G J, Sheahan B J, Liljeström P. The molecular pathogenesis of Semliki Forest virus: a model virus made useful? J Gen Virol. 1999;80:2287–2297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

