Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity
- PMID: 10888654
- PMCID: PMC112232
- DOI: 10.1128/jvi.74.15.7137-7145.2000
Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity
Abstract
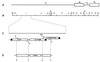
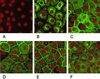
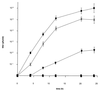
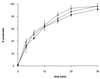
Glycoproteins homologous to the type I membrane glycoprotein B (gB) of herpes simplex virus 1 (HSV-1) are the most highly conserved glycoproteins within the family Herpesviridae and are present in members of each herpesvirus subfamily. In the alphaherpesvirus pseudorabies virus (PrV), gB is required for entry into target cells and for direct viral cell-to-cell spread. These processes, though related, appear to be distinct, and thus it was interesting to analyze whether they require different functions of gB. To this end, we established cell lines stably expressing different carboxy-terminally truncated versions of PrV gB by deleting either (i) one predicted intracytoplasmic alpha-helical domain encompassing putative YQRL and dileucine internalization signals, (ii) two predicted intracytoplasmic alpha-helical domains, (iii) the complete intracytoplasmic domain, or (iv) the intracytoplasmic domain and the transmembrane anchor region. Confocal laser scanning microscopy showed that gB derivatives lacking at least the last 29 amino acids (aa) localize close to the plasma membrane, while the full-length protein accumulates in intracellular aggregations. Trans-complementation studies with a gB-deleted PrV (PrV-gB(-)) demonstrated that the 29-aa truncated form lacking the putative internalization signals and the C-terminal alpha-helical domain (gB-008) was efficiently incorporated into PrV-gB(-) virions and efficiently complemented infectivity and cell-to-cell spread. Moreover, gB-008 exhibited an enhanced fusogenic activity. In contrast, gB proteins lacking both alpha-helical domains (gB-007), the complete intracytoplasmic domain, or the intracytoplasmic domain and transmembrane anchor were only inefficiently or not at all incorporated into PrV-gB(-) virions and did not complement infectivity. However, gB-007 was able to mediate cell-to-cell spread of PrV-gB(-). Similar phenotypes were observed when virus recombinants expressing gB-008 or gB-007, respectively, instead of wild-type gB were isolated and analyzed. Thus, our data show that internalization of gB is not required for gB incorporation into virions nor for its function in either entry or cell-to-cell spread. Moreover, they indicate different requirements for gB in these membrane fusion processes.
Figures






References
-
- Ben-Porat T, Veach R A, Ihara S. Localization of the regions of homology between the genomes of herpes simplex virus type 1 and pseudorabies virus. Virology. 1983;127:194–204. - PubMed
-
- Bold S, Ohlin M, Garten W, Radsak K. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J Gen Virol. 1996;77:2297–2302. - PubMed
-
- Bond V C, Person S, Warner S C. The isolation and characterization of mutants of herpes simplex virus type 1 that induce cell fusion. J Gen Virol. 1982;61:245–254. - PubMed
-
- Bras F, Dezélée S, Simonet B, Nguyen X, Vende P, Flamand A, Masse M J. The left border of the genomic inversion of pseudorabies virus contains genes homologous to the UL46 and UL47 genes of herpes simplex virus type 1, but no UL45 gene. Virus Res. 1999;60:29–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

