Structural determinants of murine leukemia virus reverse transcriptase that affect the frequency of template switching
- PMID: 10888659
- PMCID: PMC112237
- DOI: 10.1128/jvi.74.15.7171-7178.2000
Structural determinants of murine leukemia virus reverse transcriptase that affect the frequency of template switching
Abstract
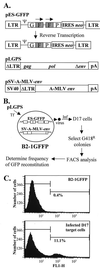
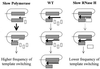
Retroviral reverse transcriptases (RTs) frequently switch templates within the same RNA or between copackaged viral RNAs to generate mutations and recombination. To identify structural elements of murine leukemia virus RT important for template switching, we developed an in vivo assay in which RT template switching within direct repeats functionally reconstituted the green fluorescent protein gene. We quantified the effect of mutations in the YXDD motif, the deoxynucleoside triphosphate binding site, the thumb domain, and the RNase H domain of RT and hydroxyurea treatment on the frequencies of template switching. Hydroxyurea treatment and some mutations in RT increased the frequency of RT template switching up to fivefold, while all of the mutations tested in the RNase H domain decreased the frequency of template switching by twofold. Based on these results, we propose a dynamic copy choice model in which both the rate of DNA polymerization and the rate of RNA degradation influence the frequency of RT template switching.
Figures
References
-
- Bavand M R, Wagner R, Richmond T J. HIV-1 reverse transcriptase: polymerization properties of the p51 homodimer compared to the p66/p51 heterodimer. Biochemistry. 1993;32:10543–10552. - PubMed
-
- Beard W A, Bebenek K, Darden T A, Li L, Prasad R, Kunkel T A, Wilson S H. Vertical-scanning mutagenesis of a critical tryptophan in the minor groove binding track of HIV-1 reverse transcriptase. Molecular nature of polymerase-nucleic acid interactions. J Biol Chem. 1998;273:30435–30442. - PubMed
-
- Beard W A, Minnick D T, Wade C L, Prasad R, Won R L, Kumar A, Kunkel T A, Wilson S H. Role of the “helix clamp” in HIV-1 reverse transcriptase catalytic cycling as revealed by alanine-scanning mutagenesis. J Biol Chem. 1996;271:12213–12220. - PubMed
-
- Beard W A, Stahl S J, Kim H R, Bebenek K, Kumar A, Strub M P, Becerra S P, Kunkel T A, Wilson S H. Structure/function studies of human immunodeficiency virus type 1 reverse transcriptase. Alanine scanning mutagenesis of an alpha-helix in the thumb subdomain. J Biol Chem. 1994;269:28091–28097. - PubMed
-
- Bebenek K, Beard W A, Casas-Finet J R, Kim H R, Darden T A, Wilson S H, Kunkel T A. Reduced frameshift fidelity and processivity of HIV-1 reverse transcriptase mutants containing alanine substitutions in helix H of the thumb subdomain. J Biol Chem. 1995;270:19516–19523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

