Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar
- PMID: 10889235
- PMCID: PMC59049
- DOI: 10.1104/pp.123.3.853
Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar
Abstract
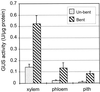
Caffeoyl coenzyme A-3-O-methyltransferase (CCoAOMT) plays an important role in lignin biosynthesis and is encoded by two genes in poplar (Populus trichocarpa). Here, we describe the expression pattern conferred by the two CCoAOMT promoters when fused to the gus-coding sequence in transgenic poplar (Populus tremula x Populus alba). Both genes were expressed similarly in xylem and differentially in phloem. In xylem, expression was preferentially observed in vessels and contact rays, whereas expression was barely detectable in storage rays and fibers, suggesting different routes to monolignol biosynthesis in the different xylem types. Furthermore, after wounding, fungal infection, and bending, the expression of both genes was induced concomitantly with de novo lignin deposition. Importantly, upon bending and leaning of the stem, the cell-specific expression pattern was lost, and both genes were expressed in all cell types of the xylem. CCoAOMT promoter activity correlated well with the presence of the CCoAOMT protein, as shown by immunolocalization. These expression data may explain, at least in part, the heterogeneity in lignin composition that is observed between cell types and upon different environmental conditions.
Figures






Similar articles
-
Secondary xylem-specific expression of caffeoyl-coenzyme A 3-O-methyltransferase plays an important role in the methylation pathway associated with lignin biosynthesis in loblolly pine.Plant Mol Biol. 1999 Jul;40(4):555-65. doi: 10.1023/a:1006244325250. Plant Mol Biol. 1999. PMID: 10480380
-
Essential role of caffeoyl coenzyme A O-methyltransferase in lignin biosynthesis in woody poplar plants.Plant Physiol. 2000 Oct;124(2):563-78. doi: 10.1104/pp.124.2.563. Plant Physiol. 2000. PMID: 11027707 Free PMC article.
-
Association of caffeoyl coenzyme A 3-O-methyltransferase expression with lignifying tissues in several dicot plants.Plant Physiol. 1997 Dec;115(4):1341-50. doi: 10.1104/pp.115.4.1341. Plant Physiol. 1997. PMID: 9414548 Free PMC article.
-
Modifications in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme A O-methyltransferase, an enzyme involved in lignin biosynthesis.J Biol Chem. 2000 Nov 24;275(47):36899-909. doi: 10.1074/jbc.M006915200. J Biol Chem. 2000. PMID: 10934215
-
Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes.Plant Cell Physiol. 2010 Jan;51(1):144-63. doi: 10.1093/pcp/pcp175. Epub 2009 Dec 8. Plant Cell Physiol. 2010. PMID: 19996151
Cited by
-
Cloning and Functional Analysis of Lignin Biosynthesis Genes Cf4CL and CfCCoAOMT in Cryptomeria fortunei.Genes (Basel). 2019 Aug 15;10(8):619. doi: 10.3390/genes10080619. Genes (Basel). 2019. PMID: 31443318 Free PMC article.
-
CCoAOMT Down-Regulation Activates Anthocyanin Biosynthesis in Petunia.Plant Physiol. 2016 Feb;170(2):717-31. doi: 10.1104/pp.15.01646. Epub 2015 Nov 30. Plant Physiol. 2016. PMID: 26620524 Free PMC article.
-
Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot.Front Plant Sci. 2015 Oct 15;6:837. doi: 10.3389/fpls.2015.00837. eCollection 2015. Front Plant Sci. 2015. PMID: 26528305 Free PMC article.
-
The seasonal activity and the effect of mechanical bending and wounding on the PtCOMT promoter in Betula pendula Roth.Plant Cell Rep. 2007 Aug;26(8):1205-14. doi: 10.1007/s00299-007-0331-x. Epub 2007 Mar 13. Plant Cell Rep. 2007. PMID: 17431633
-
MYB transcription factors are differentially expressed and regulated during secondary vascular tissue development in hybrid aspen.Plant Mol Biol. 2004 Sep;56(2):255-70. doi: 10.1007/s11103-004-3354-5. Plant Mol Biol. 2004. PMID: 15604742
References
-
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier MT, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477.
-
- Baucher M, Monties B, Van Montagu M, Boerjan W. Biosynthesis and genetic engineering of lignin. Crit Rev Plant Sci. 1998;17:125–197.
-
- Blanchette RA, Biggs AR. Defense Mechanisms of Woody Plants against Fungi (Springer Series in Wood Science). Berlin: Springer-Verlag; 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

