Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp
- PMID: 10889254
- PMCID: PMC59068
- DOI: 10.1104/pp.123.3.1047
Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp
Abstract
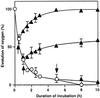
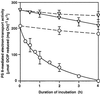
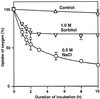
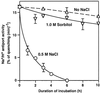
We report here that osmotic effects and ionic effects are both involved in the NaCl-induced inactivation of the photosynthetic machinery in the cyanobacterium Synechococcus sp. PCC 7942. Incubation of the cyanobacterial cells in 0.5 M NaCl induced a rapid and reversible decline and subsequent slow and irreversible loss of the oxygen-evolving activity of photosystem (PS) II and the electron transport activity of PSI. An Na(+)-channel blocker protected both PSII and PSI against the slow, but not the rapid, inactivation. The rapid decline resembled the effect of 1.0 M sorbitol. The presence of both an Na(+)-channel blocker and a water-channel blocker protected PSI and PSII against the short- and long-term effects of NaCl. Salt stress also decreased cytoplasmic volume and this effect was enhanced by the Na(+)-channel blocker. Our observations suggested that NaCl had both osmotic and ionic effects. The osmotic effect decreased the amount of water in the cytosol, rapidly increasing the intracellular concentration of salts. The ionic effect was caused by an influx of Na(+) ions through potassium/Na(+) channels that also increased concentrations of salts in the cytosol and irreversibly inactivated PSI and PSII.
Figures





References
-
- Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992;33:1215–1223.
-
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae: I. Coexistence of two photosystems in relation to Chl a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

