LXRalpha functions as a cAMP-responsive transcriptional regulator of gene expression
- PMID: 10890879
- PMCID: PMC26979
- DOI: 10.1073/pnas.100519097
LXRalpha functions as a cAMP-responsive transcriptional regulator of gene expression
Abstract
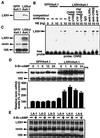
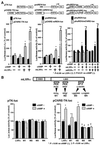
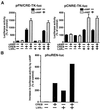
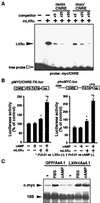
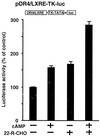
LXRalpha is a member of a nuclear receptor superfamily that regulates transcription. LXRalpha forms a heterodimer with RXRalpha, another member of this family, to regulate the expression of cholesterol 7alpha-hydroxylase by means of binding to the DR4-type cis-element. Here, we describe a function for LXRalpha as a cAMP-responsive regulator of renin and c-myc gene transcriptions by the interaction with a specific cis-acting DNA element, CNRE (an overlapping cAMP response element and a negative response element). Our previous studies showed that renin gene expression is regulated by cAMP, at least partly, through the CNRE sequence in its 5'-flanking region. This sequence is also found in c-myc and several other genes. Based on our cloning results using the yeast one-hybrid system, we discovered that the mouse homologue of human LXRalpha binds to the CNRE and demonstrated that it binds as a monomer. To define the function of LXRalpha on gene expression, we transfected the renin-producing renal As4.1 cells with LXRalpha expression plasmid. Overexpression of LXRalpha in As4.1 cells confers cAMP inducibility to reporter constructs containing the renin CNRE. After stable transfection of LXRalpha, As4.1 cells show a cAMP-inducible up-regulation of renin mRNA expression. In parallel experiments, we demonstrated that LXRalpha can also bind to the homologous CNRE in the c-myc promoter. cAMP promotes transcription through c-myc/CNRE:LXRalpha interaction in LXRalpha transiently transfected cells and increases c-myc mRNA expression in stably transfected cells. Identification of LXRalpha as a cAMP-responsive nuclear modulator of renin and c-myc expression not only has cardiovascular significance but may have generalized implication in the regulation of gene transcription.
Figures

References
-
- Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. Genes Dev. 1995;9:1033–1045. - PubMed
-
- Willy P J, Mangelsdorf D J. Genes Dev. 1997;11:289–298. - PubMed
-
- Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. - PubMed
-
- Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. - PubMed
-
- Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J M, Hammer R E, Mangelsdorf D J. Cell. 1998;93:693–704. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

