An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence
- PMID: 10890894
- PMCID: PMC27014
- DOI: 10.1073/pnas.140213697
An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence
Abstract
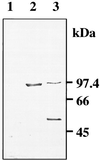
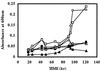
A cDNA clone encoding a lipase (lipolytic acyl hydrolase) expressed at the onset of petal senescence has been isolated by screening a cDNA expression library prepared from carnation flowers (Dianthus caryophyllus). The cDNA contains the lipase consensus sequence, ITFAGHSLGA, and encodes a 447-amino acid polypeptide with a calculated molecular mass of 50.2 kDa that appears to be a cytosolic protein. Over-expression of the clone in Escherichia coli yielded a protein of the expected molecular weight that proved capable of deesterifying fatty acids from p-nitrophenylpalmitate, tri-linolein, soybean phospholipid, and Tween in both in vitro and in situ assays of enzyme activity. The abundance of the lipase mRNA increases just as carnation flowers begin to senesce, and expression of the gene is also induced by treatment with ethylene. Southern blot analyses of carnation genomic DNA have indicated that the lipase is a single copy gene. The lipase gene is also expressed in carnation leaves and is up-regulated when the leaves are treated with ethylene. Deesterification of membrane lipids and ensuing loss of membrane structural integrity are well established early events of plant senescence, and the expression pattern of this lipase gene together with the lipolytic activity of its cognate protein indicate that it plays a fundamentally central role in mediating the onset of senescence.
Figures


References
-
- Brown J H, Paliyath G, Thompson J E. Plant Physiology: A Treatise. X. San Diego: Academic; 1991. pp. 227–275.
-
- Eze J M O, Mayak S, Thompson J E, Dumbroff E B. Physiol Plant. 1986;68:323–328.
-
- Thompson J E. In: Senescence and Aging in Plants. Noodén L D, Leopold A C, editors. San Diego: Academic; 1988. pp. 51–83.
-
- Thompson J E, Legge R L, Barber R F. New Phytol. 1987;105:317–344. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

