Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells
- PMID: 10890897
- PMCID: PMC27009
- DOI: 10.1073/pnas.140217497
Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells
Abstract
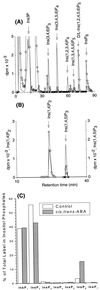
(RS)-2-cis, 4-trans-abscisic acid (ABA), a naturally occurring plant stress hormone, elicited rapid agonist-specific changes in myo-inositol hexakisphosphate (InsP(6)) measured in intact guard cells of Solanum tuberosum (n = 5); these changes were not reproduced by (RS)-2-trans, 4-trans-abscisic acid, an inactive stereoisomer of ABA (n = 4). The electrophysiological effects of InsP(6) were assessed on both S. tuberosum (n = 14) and Vicia faba (n = 6) guard cell protoplasts. In both species, submicromolar concentrations of InsP(6), delivered through the patch electrode, mimicked the inhibitory effects of ABA and internal calcium (Ca(i)(2+)) on the inward rectifying K(+) current, I(K,in), in a dose-dependent manner. Steady state block of I(K,in) by InsP(6) was reached much more quickly in Vicia (3 min at approximately 1 microM) than Solanum (20-30 min). The effects of InsP(6) on I(K,in) were specific to the myo-inositol isomer and were not elicited by other conformers of InsP(6) (e.g., scyllo- or neo-). Chelation of Ca(2+) by inclusion of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid or EGTA in the patch pipette together with InsP(6) prevented the inhibition of I(K,in), suggesting that the effect is Ca(2+) dependent. InsP(6) was approximately 100-fold more potent than Ins(1,4,5)P(3) in modulating I(K,in). Thus ABA increases InsP(6) in guard cells, and InsP(6) is a potent Ca(2+)-dependent inhibitor of I(K,in). Taken together, these results suggest that InsP(6) may play a major role in the physiological response of guard cells to ABA.
Figures



References
-
- Cosgrove D J. Inositol Phosphates, Their Chemistry, Biochemistry and Physiology. Amsterdam: Elsevier; 1980.
-
- York J D, Odom A R, Murphy R, Ives E B, Wente S R. Science. 1999;285:96–100. - PubMed
-
- Odom R A, Stahlberg A, Wente S R, York J D. Science. 2000;287:2026–2029. - PubMed
-
- Larsson O, Barker C J, Sj-oholm A, Carlqvist H, Michell R H, Bertorello A, Nilsson T, Honkanen R E, Mayr G W, Zwiller J, et al. Science. 1997;278:471–474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

