The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge
- PMID: 10890924
- PMCID: PMC26964
- DOI: 10.1073/pnas.160197797
The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge
Abstract
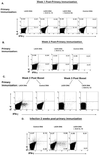
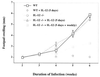
IL-12 plays a central role in both the induction and magnitude of a primary Th1 response. A critical question in designing vaccines for diseases requiring Th1 immunity such as Mycobacterium tuberculosis and Leishmania major is the requirements to sustain memory/effector Th1 cells in vivo. This report examines the role of IL-12 and antigen in sustaining Th1 responses sufficient for protective immunity to L. major after vaccination with LACK protein (LP) plus rIL-12 and LACK DNA. It shows that, after initial vaccination with LP plus rIL-12, supplemental boosting with either LP or rIL-12 is necessary but not sufficient to fully sustain long-term Th1 immunity. Moreover, endogenous IL-12 is also shown to be required for the induction, maintenance, and effector phase of the Th1 response after LACK DNA vaccination. Finally, IL-12 is required to sustain Th1 cells and control parasite growth in susceptible and resistant strains of mice during primary and secondary infection. Taken together, these data show that IL-12 is essential to sustain a sufficient number of memory/effector Th1 cells generated in vivo to mediate long-term protection to an intracellular pathogen.
Figures


References
-
- Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Science. 1995;268:563–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

