CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily
- PMID: 10891490
- PMCID: PMC86001
- DOI: 10.1128/MCB.20.15.5503-5515.2000
CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily
Abstract
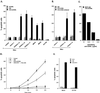
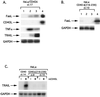
CD40, a tumor necrosis factor (TNF) receptor (TNFR) family member, conveys signals regulating diverse cellular responses, ranging from proliferation and differentiation to growth suppression and cell death. The ability of CD40 to mediate apoptosis in carcinoma cells is intriguing given the fact that the CD40 cytoplasmic C terminus lacks a death domain homology with the cytotoxic members of the TNFR superfamily, such as Fas, TNFR1, and TNF-related apoptosis-inducing ligand (TRAIL) receptors. In this study, we have probed the mechanism by which CD40 transduces death signals. Using a trimeric recombinant soluble CD40 ligand to activate CD40, we have found that this phenomenon critically depends on the membrane proximal domain (amino acids 216 to 239) but not the TNFR-associated factor-interacting PXQXT motif in the CD40 cytoplasmic tail. CD40-mediated cytotoxicity is blocked by caspase inhibitors, such as zVAD-fmk and crmA, and involves activation of caspase 8 and caspase 3. Interestingly, CD40 ligation was found to induce functional Fas ligand, TRAIL (Apo-2L) and TNF in apoptosis-susceptible carcinoma cells and to up-regulate expression of Fas. These findings identify a novel proapoptotic mechanism which is induced by CD40 in carcinoma cells and depends on the endogenous production of cytotoxic cytokines and autocrine or paracrine induction of cell death.
Figures






Similar articles
-
RIP1 has a role in CD40-mediated apoptosis in human follicular lymphoma cells.Immunobiology. 2017 Nov;222(11):998-1003. doi: 10.1016/j.imbio.2017.06.001. Epub 2017 Jun 10. Immunobiology. 2017. PMID: 28610909
-
Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells.Blood. 2005 Apr 15;105(8):3193-8. doi: 10.1182/blood-2003-10-3684. Epub 2004 Aug 31. Blood. 2005. PMID: 15339846
-
Hypericin photo-induced apoptosis involves the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and activation of caspase-8.FEBS Lett. 2001 Mar 23;493(1):26-30. doi: 10.1016/s0014-5793(01)02268-2. FEBS Lett. 2001. PMID: 11277999
-
A family of ligands for the TNF receptor superfamily.Stem Cells. 1994 Sep;12(5):440-55. doi: 10.1002/stem.5530120501. Stem Cells. 1994. PMID: 7528588 Review.
-
The role of apoptosis-inducing receptors of the tumor necrosis factor family in thyroid cancer.J Endocrinol. 2003 Aug;178(2):205-16. doi: 10.1677/joe.0.1780205. J Endocrinol. 2003. PMID: 12904168 Review.
Cited by
-
Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis.Cells. 2022 May 25;11(11):1747. doi: 10.3390/cells11111747. Cells. 2022. PMID: 35681441 Free PMC article. Review.
-
Interleukin-5 supports the expansion of fas ligand-expressing killer B cells that induce antigen-specific apoptosis of CD4(+) T cells and secrete interleukin-10.PLoS One. 2013 Aug 5;8(8):e70131. doi: 10.1371/journal.pone.0070131. Print 2013. PLoS One. 2013. PMID: 23940537 Free PMC article.
-
Human B Cell-Derived Lymphoblastoid Cell Lines Constitutively Produce Fas Ligand and Secrete MHCII(+)FasL(+) Killer Exosomes.Front Immunol. 2014 Apr 2;5:144. doi: 10.3389/fimmu.2014.00144. eCollection 2014. Front Immunol. 2014. PMID: 24765093 Free PMC article.
-
Dendritic cell subsets and implications for cancer immunotherapy.Front Immunol. 2024 Jun 5;15:1393451. doi: 10.3389/fimmu.2024.1393451. eCollection 2024. Front Immunol. 2024. PMID: 38903502 Free PMC article. Review.
-
Temporal Changes in Caspase-1 and Caspase-8 Activities Following Brain Hypoxia With and Without Src kinase Inhibition in a Piglet Animal Model.Neurochem Res. 2015 Nov;40(11):2270-9. doi: 10.1007/s11064-015-1717-8. Epub 2015 Sep 5. Neurochem Res. 2015. PMID: 26342830
References
-
- Afford S C, Randhawa S, Eliopoulos A G, Hubscher S G, Young L S, Adams D H. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface Fas ligand expression and amplifies Fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. - PMC - PubMed
-
- Baker M P, Eliopoulos A G, Young L S, Armitage R J, Gregory C D, Gordon J. Prolonged phenotypic, functional and molecular changes in group I Burkitt lymphoma cells on short term exposure to CD40 ligand. Blood. 1998;92:2830–2843. - PubMed
-
- Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. - PubMed
-
- Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A B. Human CD8(+) T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. - PubMed
-
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
