Long-distance control of origin choice and replication timing in the human beta-globin locus are independent of the locus control region
- PMID: 10891496
- PMCID: PMC86017
- DOI: 10.1128/MCB.20.15.5581-5591.2000
Long-distance control of origin choice and replication timing in the human beta-globin locus are independent of the locus control region
Abstract
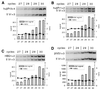
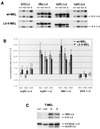
DNA replication in the human beta-globin locus is subject to long-distance regulation. In murine and human erythroid cells, the human locus replicates in early S phase from a bidirectional origin located near the beta-globin gene. This Hispanic thalassemia deletion removes regulatory sequences located over 52 kb from the origin, resulting in replication of the locus from a different origin, a shift in replication timing to late S phase, adoption of a closed chromatin conformation, and silencing of globin gene expression in murine erythroid cells. The sequences deleted include nuclease-hypersensitive sites 2 to 5 (5'HS2-5) of the locus control region (LCR) plus an additional 27-kb upstream region. We tested a targeted deletion of 5'HS2-5 in the normal chromosomal context of the human beta-globin locus to determine the role of these elements in replication origin choice and replication timing. We demonstrate that the 5'HS2-5-deleted locus initiates replication at the appropriate origin and with normal timing in murine erythroid cells, and therefore we conclude that 5'HS2-5 in the classically defined LCR do not control replication in the human beta-globin locus. Recent studies also show that targeted deletion of 5'HS2-5 results in a locus that lacks globin gene expression yet retains an open chromatin conformation. Thus, the replication timing of the locus is closely correlated with nuclease sensitivity but not globin gene expression.
Figures





References
-
- Aladjem M I, Groudine M, Brody L L, Dieken E S, Fournier R E, Wahl G M, Epner E M. Participation of the human beta-globin locus control region in initiation of DNA replication. Science. 1995;270:815–819. - PubMed
-
- Aladjem M I, Rodewald L W, Kolman J L, Wahl G M. Genetic dissection of a mammalian replicator in the human beta-globin locus. Science. 1998;281:1005–1009. - PubMed
-
- Bender M, Bulger M, Close J, Groudine M. Globin gene switching and DNaseI sensitivity of the endogenous β globin locus in mice do not require the locus control region. Mol Cell. 2000;5:387–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
