Mutations in conserved regions of the predicted RAG2 kelch repeats block initiation of V(D)J recombination and result in primary immunodeficiencies
- PMID: 10891502
- PMCID: PMC86034
- DOI: 10.1128/MCB.20.15.5653-5664.2000
Mutations in conserved regions of the predicted RAG2 kelch repeats block initiation of V(D)J recombination and result in primary immunodeficiencies
Abstract
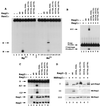
The V(D)J recombination reaction is composed of multiple nucleolytic processing steps mediated by the recombination-activating proteins RAG1 and RAG2. Sequence analysis has suggested that RAG2 contains six kelch repeat motifs that are predicted to form a six-bladed beta-propeller structure, with the second beta-strand of each repeat demonstrating marked conservation both within and between kelch repeat-containing proteins. Here we demonstrate that mutations G95R and DeltaI273 within the predicted second beta-strand of repeats 2 and 5 of RAG2 lead to immunodeficiency in patients P1 and P2. Green fluorescent protein fusions with the mutant proteins reveal appropriate localization to the nucleus. However, both mutations reduce the capacity of RAG2 to interact with RAG1 and block recombination signal cleavage, therefore implicating a defect in the early steps of the recombination reaction as the basis of the clinical phenotype. The present experiments, performed with an extensive panel of site-directed mutations within each of the six kelch motifs, further support the critical role of both hydrophobic and glycine-rich regions within the second beta-strand for RAG1-RAG2 interaction and recombination signal recognition and cleavage. In contrast, multiple mutations within the variable-loop regions of the kelch repeats had either mild or no effects on RAG1-RAG2 interaction and hence on the ability to mediate recombination. In all, the data demonstrate a critical role of the RAG2 kelch repeats for V(D)J recombination and highlight the importance of the conserved elements of the kelch motif.
Figures






References
-
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. - PubMed
-
- Agrawal A, Eastman Q M, Schatz D G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. - PubMed
-
- Aidinis V, Dias D C, Gomez C A, Bhattacharyya D, Spanopoulou E, Santagata S. Definition of minimal domains of interaction within the recombination-activating genes 1 and 2 recombinase complex. J Immunol. 2000;164:5826–5832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
