The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control
- PMID: 10893258
- PMCID: PMC2185565
- DOI: 10.1083/jcb.150.1.77
The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control
Abstract
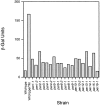
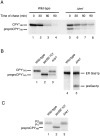
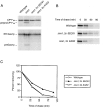
The unfolded protein response (UPR) is an intracellular signaling pathway that relays signals from the lumen of the ER to activate target genes in the nucleus. We devised a genetic screen in the yeast Saccharomyces cerevisiae to isolate mutants that are dependent on activation of the pathway for viability. Using this strategy, we isolated mutants affecting various aspects of ER function, including protein translocation, folding, glycosylation, glycosylphosphatidylinositol modification, and ER-associated protein degradation (ERAD). Extending results gleaned from the genetic studies, we demonstrate that the UPR regulates trafficking of proteins at the translocon to balance the needs of biosynthesis and ERAD. The approach also revealed connections of the UPR to other regulatory pathways. In particular, we identified SON1/RPN4, a recently described transcriptional regulator for genes encoding subunits of the proteasome. Our genetic strategy, therefore, offers a powerful means to provide insight into the physiology of the UPR and to identify novel genes with roles in many aspects of secretory and membrane protein biogenesis.
Figures




References
-
- Biederer T., Volkwein C., Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. - PubMed
-
- Boeke J.D., LaCroute F., Fink G.R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast5-fluoro-orotic acid resistance. Mol. Gen. Genet. 1984;197:345–346. - PubMed
-
- Brodsky J.L., McCracken A.A. ER protein quality control and proteasome-mediated protein degradation. Sem. Cell Dev. Biol. 1999;10:501–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

