Molecular basis for Rab prenylation
- PMID: 10893259
- PMCID: PMC2185574
- DOI: 10.1083/jcb.150.1.89
Molecular basis for Rab prenylation
Abstract
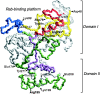
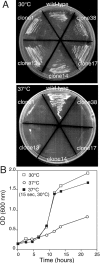
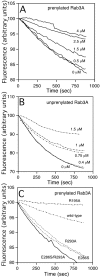
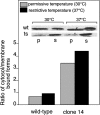
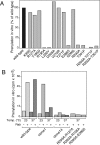
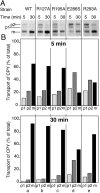
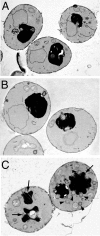
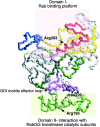
Rab escort proteins (REP) 1 and 2 are closely related mammalian proteins required for prenylation of newly synthesized Rab GTPases by the cytosolic heterodimeric Rab geranylgeranyl transferase II complex (RabGG transferase). REP1 in mammalian cells is the product of the choroideremia gene (CHM). CHM/REP1 deficiency in inherited disease leads to degeneration of retinal pigmented epithelium and loss of vision. We now show that amino acid residues required for Rab recognition are critical for function of the yeast REP homologue Mrs6p, an essential protein that shows 50% homology to mammalian REPs. Mutant Mrs6p unable to bind Rabs failed to complement growth of a mrs6Delta null strain and were found to be dominant inhibitors of growth in a wild-type MRS6 strain. Mutants were identified that did not affect Rab binding, yet prevented prenylation in vitro and failed to support growth of the mrs6Delta null strain. These results suggest that in the absence of Rab binding, REP interaction with RabGG transferase is maintained through Rab-independent binding sites, providing a molecular explanation for the kinetic properties of Rab prenylation in vitro. Analysis of the effects of thermoreversible temperature-sensitive (mrs6(ts)) mutants on vesicular traffic in vivo showed prenylation activity is only transiently required to maintain normal growth, a result promising for therapeutic approaches to disease.
Figures



References
-
- Alexandrov K., Simon I., Yurchenko V., Iakovenko A., Rostkova E., Scheidig A.J., Goody R.S. Characterization of the ternary complex between Rab7, REP-1 and Rab geranylgeranyl transferase. Eur. J. Biochem. 1999;265:160–170. - PubMed
-
- Anant J.S., Desnoyers L., Machius M., Demeler B., Hansen J.C., Westover K.D., Deisenhofer J., Seabra M.C. Mechanism of Rab geranylgeranylationformation of the catalytic ternary complex. Biochemistry. 1998;37:12559–12568. - PubMed
-
- Andres D.A., Seabra M.C., Brown M.S., Armstrong S.A., Smeland T.E., Cremers F.P.M., Goldstein J.L. cDNA cloning of component A of rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73:1091–1099. - PubMed
-
- Cadwell R.C., Joyce G.F. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 1992;2:28–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

