Glutamate slows axonal transport of neurofilaments in transfected neurons
- PMID: 10893265
- PMCID: PMC2185569
- DOI: 10.1083/jcb.150.1.165
Glutamate slows axonal transport of neurofilaments in transfected neurons
Abstract
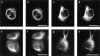
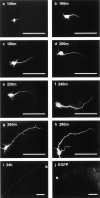
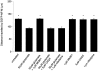
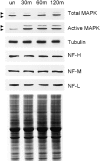
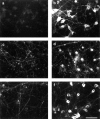
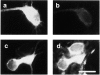
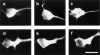
Neurofilaments are transported through axons by slow axonal transport. Abnormal accumulations of neurofilaments are seen in several neurodegenerative diseases, and this suggests that neurofilament transport is defective. Excitotoxic mechanisms involving glutamate are believed to be part of the pathogenic process in some neurodegenerative diseases, but there is currently little evidence to link glutamate with neurofilament transport. We have used a novel technique involving transfection of the green fluorescent protein-tagged neurofilament middle chain to measure neurofilament transport in cultured neurons. Treatment of the cells with glutamate induces a slowing of neurofilament transport. Phosphorylation of the side-arm domains of neurofilaments has been associated with a slowing of neurofilament transport, and we show that glutamate causes increased phosphorylation of these domains in cell bodies. We also show that glutamate activates members of the mitogen-activated protein kinase family, and that these kinases will phosphorylate neurofilament side-arm domains. These results provide a molecular framework to link glutamate excitotoxicity with neurofilament accumulation seen in some neurodegenerative diseases.
Figures



References
-
- Aplin A.E., Gibb G.M., Jacobsen J.S., Gallo J.-M., Anderton B.H. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3β. J. Neurochem. 1996;67:699–707. - PubMed
-
- Archer D.R., Watson D.F., Griffin J.W. Phosphorylation-dependent immunoreactivity of neurofilaments and the rate of slow axonal transport in the central and peripheral axons of the rat dorsal root ganglia. J. Neurochem. 1994;62:1119–1125. - PubMed
-
- Asahara H., Taniwaki T., Ohyagi Y., Yamada T., Kira J. Glutamate enhances phosphorylation of neurofilaments in cerebellar granule cell culture. J. Neurol. Sci. 1999;171:84–87. - PubMed
-
- Baas P.W., Brown A. Slow axonal transportthe polymer transport model. Trends Cell Biol. 1997;7:380–384. - PubMed
-
- Bading H., Greenberg M.E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

