The actin cytoskeleton in store-mediated calcium entry
- PMID: 10896713
- PMCID: PMC2270006
- DOI: 10.1111/j.1469-7793.2000.t01-2-00221.x
The actin cytoskeleton in store-mediated calcium entry
Abstract
Store-mediated Ca2+ entry is the main pathway for Ca2+ influx in platelets and many other cells. Several hypotheses have considered both direct and indirect coupling mechanisms between the endoplasmic reticulum and the plasma membrane. Here we pay particular attention to new insights into the regulation of store-mediated Ca2+ entry: the role of the cytoskeleton in a secretion-like coupling model. In this model, Ca2+ entry may be mediated by a reversible trafficking and coupling of the endoplasmic reticulum with the plasma membrane, that shows close parallels to the events mediating secretion. As with secretion, the actin cytoskeleton plays an inhibitory role in the activation of Ca2+ entry by preventing the approach and coupling of the endoplasmic reticulum with the plasma membrane, making cytoskeletal remodelling a key event in the activation of Ca2+ entry. We also review recent advances investigating the regulation of store-mediated Ca2+ entry by small GTPases and phosphoinositides, which might be involved in the store-mediated Ca2+ entry pathway through roles in the remodelling of the cytoskeleton.
Figures

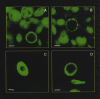
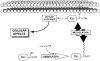

References
-
- Álvarez J, Montero M, García-Sancho J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB Journal. 1992;6:786–792. - PubMed
-
- Ando S, Kaibuchi K, Sasaki T, Hiraoka K, Nishiyama T, Mizuno T, Asada M, Nunoi H, Matsuda I, Matsuura Y, Polakis P, McCormick F, Takai Y. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. Journal of Biological Chemistry. 1992;267:25709–25713. - PubMed
-
- Bird GStJ, Putney JW., Jr Inhibition of thapsigargin-induced calcium entry by microinjected guanine nucleotide analogues. Evidence for the involvement of a small G-protein in capacitative calcium entry. Journal of Biological Chemistry. 1993;268:21486–21488. - PubMed
-
- Brown BL, Walker SW, Tomlinson S. Calcium calmodulin and hormone secretion. Clinical Endocrinology. 1985;23:201–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

