Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria
- PMID: 10898680
- PMCID: PMC90018
- DOI: 10.1128/AAC.44.8.2086-2092.2000
Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria
Abstract
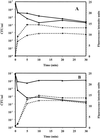
Antimicrobial cationic peptides are ubiquitous in nature and are thought to be a component of the first line of defense against infectious agents. It is widely believed that the killing mechanism of these peptides on bacteria involves an interaction with the cytoplasmic membrane. Cationic peptides from different structural classes were used in experiments with Staphylococcus aureus and other medically important gram-positive bacteria to gain insight into the mechanism of action. The membrane potential-sensitive fluorophore dipropylthiacarbocyanine was used to assess the interactions of selected antimicrobial peptides with the cytoplasmic membrane of S. aureus. Study of the kinetics of killing and membrane depolarization showed that, at early time points, membrane depolarization was incomplete, even when 90% or more of the bacteria had been killed. CP26, a 26-amino-acid alpha-helical peptide with a high MIC against S. aureus, still had the ability to permeabilize the membrane. Cytoplasmic-membrane permeabilization was a widespread ability and an action that may be necessary for reaching an intracellular target but in itself did not appear to be the killing mechanism. Transmission electron microscopy of S. aureus and Staphylococcus epidermidis treated with CP29 (a 26-amino-acid alpha-helical peptide), CP11CN (a 13-amino-acid, proline- and tryptophan-rich peptide), and Bac2A-NH(2) (a linearized version of the 12-amino-acid loop peptide bactenecin) showed variability in effects on bacterial structure. Mesosome-like structures were seen to develop in S. aureus, whereas cell wall effects and mesosomes were seen with S. epidermidis. Nuclear condensation and abherrent septation were occasionally seen in S. epidermidis. Our experiments indicated that these peptides vary in their mechanisms of action and that the mechanism of action likely does not solely involve cytoplasmic-membrane permeabilization.
Figures




References
-
- Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 52–111.
-
- Beveridge T J. The structure of bacteria. In: Leadbetter E R, Poindexter J S, editors. Bacteria in nature: a treatise on the interaction of bacteria and their habitats. Vol. 3. New York, N.Y: Plenum Publishing Co.; 1989. pp. 1–65.
-
- Bierbaum G, Sahl H G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. - PubMed
-
- Castle M, Nazarian A, Yi S S, Tempst P. Lethal effects of apidaecin on Escherichia coli involve sequential molecular interactions with diverse targets. J Biol Chem. 1999;274:32555–32564. - PubMed
-
- Cociancich S, Ghazi A, Hoffman J A, Hetrus C, Letellier C. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993;268:19239–19245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

