Disruption of the ARF transcriptional activator DMP1 facilitates cell immortalization, Ras transformation, and tumorigenesis
- PMID: 10898794
- PMCID: PMC316790
Disruption of the ARF transcriptional activator DMP1 facilitates cell immortalization, Ras transformation, and tumorigenesis
Abstract
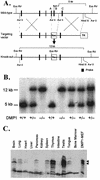
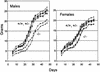
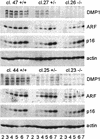
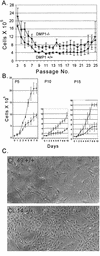
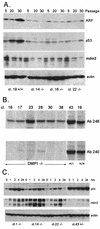
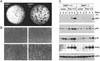
The DMP1 transcription factor induces the ARF tumor suppressor gene in mouse fibroblasts, leading to cell cycle arrest in a p53-dependent manner. We disrupted sequences encoding the DNA-binding domain of DMP1 in mouse embryonic stem cells and derived animals lacking the functional protein. DMP1-null animals are small at birth, and males develop more slowly than their wild-type littermates. Some adult animals exhibit seizures and/or obstuctive uropathy, each of unknown cause. The growth of explanted DMP1-null mouse embryo fibroblasts (MEFs) is progressively retarded as cells are passaged in culture on defined transfer protocols; but, unlike the behavior of normal cells, p19(ARF), Mdm2, and p53 levels remain relatively low and DMP1-null MEFs do not senesce. Whereas the establishment of cell lines from MEFs is usually always accompanied by either p53 or ARF loss of function, continuously passaged DMP1-null cells readily give rise to established 3T3 and 3T9 cell lines that retain wild-type ARF and functional p53 genes. Early-passage DMP1-null cells, like MEFs from either ARF-null or p53-null mice, can be morphologically transformed by oncogenic Ha-Ras (Val-12) alone. Splenic lymphocytes harvested from both DMP1-null and ARF-null mice exhibit enhanced proliferative responses in long-term cultures when stimulated to divide with antibody to CD3 and interleukin-2. Although only 1 of 40 DMP1-null animals spontaneously developed a tumor in the first year of life, neonatal treatment with dimethylbenzanthracene or ionizing radiation induced tumors of various histologic types that were not observed in similarly treated DMP1(+/+) animals. Karyotypic analyses of MEFs and lymphomas from DMP1-null animals revealed pseudodiploid chromosome numbers, consistent with the retention of wild-type p53. Together, these data suggest that ARF function is compromised, but not eliminated, in animals lacking functional DMP1.
Figures

References
-
- Babcock VI, Southam CM. Obstructive uropathy in laboratory mice. Proc Soc Exp Biol Med. 1965;120:580–581. - PubMed
-
- Bates S, Phillips AC, Clarke P, Stott F, Peters G, Ludwig RL, Vousden KH. E2F-1 regulation of p14ARF links pRB and p53. Nature. 1998;395:124–125. - PubMed
-
- Bodner SM, Naeve CW, Rakestraw KM, Jones BG, Valentine VA, Valentine MB, Luthardt FW, Willman CL, Raimondi SC, Downing JR, et al. Cloning and chromosomal localization of the gene encoding human cyclin D-binding Myb-like protein (hDMP1) Gene. 1999;229:223–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
