Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection
- PMID: 10899110
- PMCID: PMC313993
- DOI: 10.1093/emboj/19.14.3556
Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection
Abstract
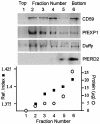
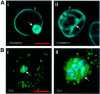
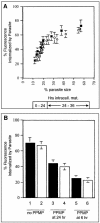
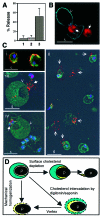
Erythrocytes, which are incapable of endocytosis or phagocytosis, can be infected by the malaria parasite Plasmodium falciparum. We find that a transmembrane protein (Duffy), glycosylphosphatidylinositol (GPI)-anchored and cytoplasmic proteins, associated with detergent-resistant membranes (DRMs) that are characteristic of microdomains in host cell membranes, are internalized by vacuolar parasites, while the major integral membrane and cytoskeletal proteins are not. The internalized host proteins and a plasmodial transmembrane resident parasitophorous vacuolar membrane (PVM) protein are detected in DRMs associated with vacuolar parasites. This is the first report of a host transmembrane protein being recruited into an apicomplexan vacuole and of the presence of vacuolar DRMs; it establishes that integral association does not preclude protein internalization into the P.FALCIPARUM: vacuole. Rather, as shown for Duffy, intracellular accumulation occurs at the same rate as that seen for a DRM-associated GPI-anchored protein. Furthermore, novel mechanisms regulated by the DRM lipids, sphingomyelin and cholesterol, mediate (i) the uptake of host DRM proteins and (ii) maintenance of the intracellular vacuole in the non-endocytic red cell, which may have implications for intracellular parasitism and pathogenesis.
Figures


References
-
- Anderson R. (1998) The caveolae membrane system. Annu. Rev. Biochem., 67, 199–225. - PubMed
-
- Barnwell J.W. (1990) Vesicle-mediated transport of membrane and proteins in malaria-infected erythrocytes. Blood Cells, 16, 379–395. - PubMed
-
- Brown D.A. and London,E. (1998a) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. - PubMed
-
- Brown D.A. and London,E. (1998b) Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol., 164, 103–114. - PubMed
-
- Brown D.A. and Rose,J.,K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

