Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae
- PMID: 10899840
- PMCID: PMC98342
- DOI: 10.1128/IAI.68.8.4430-4440.2000
Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae
Abstract
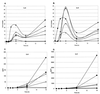
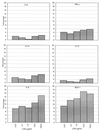
Nontypeable Haemophilus influenzae (NTHi) causes repeated respiratory infections in patients with chronic lung diseases. These infections are characterized by a brisk inflammatory response which results in the accumulation of polymorphonucleated cells in the lungs and is dependent on the expression and secretion of proinflammatory cytokines. We hypothesize that multiple NTHi molecules, including lipooligosaccharide (LOS), mediate cellular interactions with respiratory epithelial cells, leading to the production of proinflammatory cytokines. To address this hypothesis, we exposed 9HTEo- human tracheal epithelial cells to NTHi and compared the resulting profiles of cytokine gene expression and secretion using multiprobe RNase protection assays and enzyme-linked immunosorbent assays (ELISA), respectively. Dose-response experiments demonstrated a maximum stimulation of most cytokines tested, using a ratio of 100 NTHi bacterial cells to 1 9HTEo- tracheal epithelial cell. Compared with purified LOS, NTHi bacterial cells stimulated 3.6- and 4.5-fold increases in epithelial cell expression of interleukin-8 (IL-8) and IL-6 genes, respectively. Similar results were seen with epithelial cell macrophage chemotactic protein 1, IL-1alpha, IL-1beta, and tumor necrosis factor alpha expression. Polymyxin B completely inhibited LOS stimulation but only partially reduced NTHi whole cell stimulation. Taken together, these results suggest that multiple bacterial molecules including LOS contribute to the NTHi stimulation of respiratory epithelial cell cytokine production. Moreover, no correlation was seen between NTHi adherence to epithelial cells mediated by hemagglutinating pili, Hia, HMW1, HMW2, and Hap and epithelial cytokine secretion. These data suggest that bacterial molecules beyond previously described NTHi cell surface adhesins and LOS play a role in the induction of proinflammatory cytokines from respiratory epithelial cells.
Figures




References
-
- Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. - PubMed
-
- Bonfield T, Panuska J, Konstan M, Hilliard K, Hilliard J, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. - PubMed
-
- Bonfield T, Konstan M, Burfeind P, Panuska J, Hilliard J, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. - PubMed
-
- Bresser P, van Alphen L, Habets F J M, Hart A A M, Dankert J, Jansen H M, Lutter R. Persisting Haemophilus influenzae strains induce lower levels of interleukin-6 and interleukin-8 in H292 lung epithelial cells than nonpersisting strains. Eur Respir J. 1997;10:2319–2326. - PubMed
-
- Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine III R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

