Variability of outer membrane protein P1 and its evaluation as a vaccine candidate against experimental otitis media due to nontypeable Haemophilus influenzae: an unambiguous, multifaceted approach
- PMID: 10899849
- PMCID: PMC98360
- DOI: 10.1128/IAI.68.8.4505-4517.2000
Variability of outer membrane protein P1 and its evaluation as a vaccine candidate against experimental otitis media due to nontypeable Haemophilus influenzae: an unambiguous, multifaceted approach
Abstract
Candidate vaccine antigens for preventing otitis media caused by nontypeable Haemophilus influenzae (NTHI) should possess one or more conserved epitopes. We sought to evaluate the candidacy of P1, a surface-expressed outer membrane protein knowing that this antigen is subject to diversifying selection. Therefore, we selected NTHI strains from among >500 phylogenically variant isolates representative of the diversity found in natural populations of H. influenzae. Twenty-three variants of P1 (</=95% similarity) were identified among 42 strains. When chinchillas were immunized with recombinant P1 (rP1) obtained from one of these isolates (BCH-3), all animals developed antibodies specific for rP1. Immunized animals were protected against disease when challenged with BCH-3, but not with an ompP1 mutant of BCH-3 or a strain (BCH-2) possessing a heterologous P1 (91% identity). We conclude that (i) while P1 induces protection against NTHI-mediated otitis media, development of a polyvalent vaccine reflecting the variability of P1 would be necessary to construct an efficacious vaccine and (ii) use of a phylogenically characterized collection of representative isolates in concert with gene sequencing, cloning, gene inactivation, and animal testing offers an efficient, rational, and rigorous strategy for evaluating the potential problems associated with variability of vaccine targets and specificity of related immune responses.
Figures

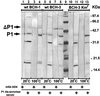



References
-
- Barcak G J, Chandler M S, Redfield R J, Tomb J F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

