Novel apoptosis-inducing activity in Bacteroides forsythus: a comparative study with three serotypes of Actinobacillus actinomycetemcomitans
- PMID: 10899863
- PMCID: PMC98390
- DOI: 10.1128/IAI.68.8.4611-4615.2000
Novel apoptosis-inducing activity in Bacteroides forsythus: a comparative study with three serotypes of Actinobacillus actinomycetemcomitans
Abstract
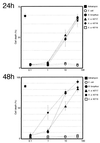
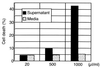
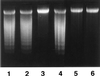
Bacteroides forsythus, which has been reported to be associated with periodontitis but has not been recognized as a key pathogen, was found to induce cytolytic activity against HL-60 and other human leukemic cells. This cytolytic activity was demonstrated according to three different criteria: (i) loss of both mitochondrial membrane potential and membrane integrity in cells treated with bacterial extracts and then with Rh123 and propidium iodide, respectively, as demonstrated by flow cytometry; (ii) damage to cytoplasmic membrane, as revealed by scanning electron microscopy (SEM); and (iii) DNA ladder formation and activation of caspase-3. These results indicate that B. forsythus produced an apoptosis-inducing factor(s) found to be composed of protein as judged by heat and trypsin sensitivity. In addition to extracts from B. forsythus, the culture supernatant of this bacterium has the ability to induce a cytolytic effect against peripheral white blood cells, especially lymphocytes. For comparison with B. forsythus, the same analyses were applied to two strains with different serotypes of Actinobacillus actinomycetemcomitans, serotypes a (ATCC 43717) and c (ATCC 43719), in addition to previously reported apoptosis-inducing serotype b (ATCC 43718), which was used as a positive control. The strains of A. actinomycetemcomitans serotypes a and b induced apoptosis in HL-60 cells as judged by the above three criteria but to a slightly lesser extent than did B. forsythus, while the serotype c strain produced apoptosis to a negligible extent. Detailed SEM images showed that the A. actinomycetemcomitans serotype a strain induced large-pore formation and the serotype b strain produced small pores with typical blebbing, while B. forsythus induced severe membrane ruffling. Further DNA ladder formation and caspase-3 activation were observed in the serotype a and b strains but not in the serotype c strain. The present paper is the first report of a protein factor(s) from B. forsythus and the A. actinomycetemcomitans serotype a strain which induces apoptotic cell death.
Figures


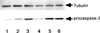
References
-
- Arab S, Murakami M, Dirks P, Boyd B, Hubbard S L, Lingwood C A, Rutka J T. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J Neurooncol. 1998;40:137–150. - PubMed
-
- Bertrand S, Berger R, Philip T, Bernheim A, Bryon P A, Bertoglio J, Dore J F, Brunat-Mentigny M, Lenoir G M. Variant translocation in a non endemic case of Burkitt's lymphoma: t(8;22) in an Epstein-Barr virus negative tumor and in a derived cell line. Eur J Cancer. 1981;17:577–584. - PubMed
-
- Collins S J, Gallo R C, Gallagher R E. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 1977;270:347–349. - PubMed
-
- Fives-Taylor P M, Meyer D H, Mintz K P, Brisette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;29:136–167. - PubMed
-
- Gewirtz D A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

